Design and analysis of rhesus cytomegalovirus IL-10 mutants as a model for novel vaccines against human cytomegalovirus
- PMID: 22132227
- PMCID: PMC3221699
- DOI: 10.1371/journal.pone.0028127
Design and analysis of rhesus cytomegalovirus IL-10 mutants as a model for novel vaccines against human cytomegalovirus
Abstract
Background: Human cytomegalovirus (HCMV) expresses a viral ortholog (CMVIL-10) of human cellular interleukin-10 (cIL-10). Despite only ∼26% amino acid sequence identity, CMVIL-10 exhibits comparable immunosuppressive activity with cIL-10, attenuates HCMV antiviral immune responses, and contributes to lifelong persistence within infected hosts. The low sequence identity between CMVIL-10 and cIL-10 suggests vaccination with CMVIL-10 may generate antibodies that specifically neutralize CMVIL-10 biological activity, but not the cellular cytokine, cIL-10. However, immunization with functional CMVIL-10 might be detrimental to the host because of its immunosuppressive properties.
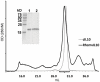
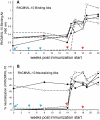
Methods and findings: Structural biology was used to engineer biologically inactive mutants of CMVIL-10 that would, upon vaccination, elicit a potent immune response to the wild-type viral cytokine. To test the designed proteins, the mutations were incorporated into the rhesus cytomegalovirus (RhCMV) ortholog of CMVIL-10 (RhCMVIL-10) and used to vaccinate RhCMV-infected rhesus macaques. Immunization with the inactive RhCMVIL-10 mutants stimulated antibodies against wild-type RhCMVIL-10 that neutralized its biological activity, but did not cross-react with rhesus cellular IL-10.
Conclusion: This study demonstrates an immunization strategy to neutralize RhCMVIL-10 biological activity using non-functional RhCMVIL-10 antigens. The results provide the methodology for targeting CMVIL-10 in vaccine, and therapeutic strategies, to nullify HCMV's ability to (1) skew innate and adaptive immunity, (2) disseminate from the site of primary mucosal infection, and (3) establish a lifelong persistent infection.
Conflict of interest statement
Figures


Similar articles
-
Vaccination against a virus-encoded cytokine significantly restricts viral challenge.J Virol. 2013 Nov;87(21):11323-31. doi: 10.1128/JVI.01925-13. Epub 2013 Aug 14. J Virol. 2013. PMID: 23946461 Free PMC article.
-
Host immune responses to a viral immune modulating protein: immunogenicity of viral interleukin-10 in rhesus cytomegalovirus-infected rhesus macaques.PLoS One. 2012;7(5):e37931. doi: 10.1371/journal.pone.0037931. Epub 2012 May 24. PLoS One. 2012. PMID: 22655082 Free PMC article.
-
Exploitation of Interleukin-10 (IL-10) Signaling Pathways: Alternate Roles of Viral and Cellular IL-10 in Rhesus Cytomegalovirus Infection.J Virol. 2016 Oct 14;90(21):9920-9930. doi: 10.1128/JVI.00635-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27558431 Free PMC article.
-
Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus.Adv Virus Res. 2008;72:207-26. doi: 10.1016/S0065-3527(08)00405-3. Adv Virus Res. 2008. PMID: 19081492 Review.
-
Exploiting viral natural history for vaccine development.Med Microbiol Immunol. 2015 Jun;204(3):255-62. doi: 10.1007/s00430-015-0406-1. Epub 2015 Mar 21. Med Microbiol Immunol. 2015. PMID: 25794555 Free PMC article. Review.
Cited by
-
Vaccination against a virus-encoded cytokine significantly restricts viral challenge.J Virol. 2013 Nov;87(21):11323-31. doi: 10.1128/JVI.01925-13. Epub 2013 Aug 14. J Virol. 2013. PMID: 23946461 Free PMC article.
-
Modulation of dendritic cell functions by viral IL-10 encoded by human cytomegalovirus.Front Microbiol. 2014 Jul 4;5:337. doi: 10.3389/fmicb.2014.00337. eCollection 2014. Front Microbiol. 2014. PMID: 25071749 Free PMC article. Review.
-
Host immune responses to a viral immune modulating protein: immunogenicity of viral interleukin-10 in rhesus cytomegalovirus-infected rhesus macaques.PLoS One. 2012;7(5):e37931. doi: 10.1371/journal.pone.0037931. Epub 2012 May 24. PLoS One. 2012. PMID: 22655082 Free PMC article.
-
Neutralization of rhesus cytomegalovirus IL-10 reduces horizontal transmission and alters long-term immunity.Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):13036-13041. doi: 10.1073/pnas.1903317116. Epub 2019 Jun 12. Proc Natl Acad Sci U S A. 2019. PMID: 31189602 Free PMC article.
-
Exploitation of Interleukin-10 (IL-10) Signaling Pathways: Alternate Roles of Viral and Cellular IL-10 in Rhesus Cytomegalovirus Infection.J Virol. 2016 Oct 14;90(21):9920-9930. doi: 10.1128/JVI.00635-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27558431 Free PMC article.
References
-
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

