Functional consequences of prolactin signalling in endothelial cells: a potential link with angiogenesis in pathophysiology?
- PMID: 22128761
- PMCID: PMC3822974
- DOI: 10.1111/j.1582-4934.2011.01499.x
Functional consequences of prolactin signalling in endothelial cells: a potential link with angiogenesis in pathophysiology?
Abstract
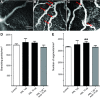
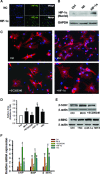
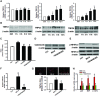
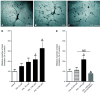
Prolactin is best known as the polypeptide anterior pituitary hormone, which regulates the development of the mammary gland. However, it became clear over the last decade that prolactin contributes to a broad range of pathologies, including breast cancer. Prolactin is also involved in angiogenesis via the release of pro-angiogenic factors by leukocytes and epithelial cells. However, whether prolactin also influences endothelial cells, and whether there are functional consequences of prolactin-induced signalling in the perspective of angiogenesis, remains so far elusive. In the present study, we show that prolactin induces phosphorylation of ERK1/2 and STAT5 and induces tube formation of endothelial cells on Matrigel. These effects are blocked by a specific prolactin receptor antagonist, del1-9-G129R-hPRL. Moreover, in an in vivo model of the chorioallantoic membrane of the chicken embryo, prolactin enhances vessel density and the tortuosity of the vasculature and pillar formation, which are hallmarks of intussusceptive angiogenesis. Interestingly, while prolactin has only little effect on endothelial cell proliferation, it markedly stimulates endothelial cell migration. Again, migration was reverted by del1-9-G129R-hPRL, indicating a direct effect of prolactin on its receptor. Immunohistochemistry and spectral imaging revealed that the prolactin receptor is present in the microvasculature of human breast carcinoma tissue. Altogether, these results suggest that prolactin may directly stimulate angiogenesis, which could be one of the mechanisms by which prolactin contributes to breast cancer progression, thereby providing a potential tool for intervention.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures


Similar articles
-
Prolactin antagonist-endostatin fusion protein as a targeted dual-functional therapeutic agent for breast cancer.Cancer Res. 2003 Jul 1;63(13):3598-604. Cancer Res. 2003. PMID: 12839947
-
Porphyromonas gingivalis, periodontal pathogen, lipopolysaccharide induces angiogenesis via extracellular signal-regulated kinase 1/2 activation in human vascular endothelial cells.Arch Pharm Res. 2007 Jan;30(1):34-42. doi: 10.1007/BF02977776. Arch Pharm Res. 2007. PMID: 17328240
-
Prolactin receptor antagonism uncouples lipids from atherosclerosis susceptibility.J Endocrinol. 2014 Sep;222(3):341-50. doi: 10.1530/JOE-14-0343. Epub 2014 Jul 25. J Endocrinol. 2014. PMID: 25063756
-
Use of prolactin receptor antagonist to better understand prolactin regulation of pituitary homeostasis.Neuroendocrinology. 2013;98(3):171-9. doi: 10.1159/000354701. Epub 2013 Sep 19. Neuroendocrinology. 2013. PMID: 23969780 Review.
-
A positive feedback loop between prolactin and STAT5 promotes angiogenesis.Adv Exp Med Biol. 2015;846:265-80. doi: 10.1007/978-3-319-12114-7_12. Adv Exp Med Biol. 2015. PMID: 25472543 Review.
Cited by
-
STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis.J Biol Chem. 2013 Jul 19;288(29):21184-21196. doi: 10.1074/jbc.M113.481119. Epub 2013 Jun 2. J Biol Chem. 2013. PMID: 23729680 Free PMC article.
-
Principles of the prolactin/vasoinhibin axis.Am J Physiol Regul Integr Comp Physiol. 2015 Nov 15;309(10):R1193-203. doi: 10.1152/ajpregu.00256.2015. Epub 2015 Aug 26. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26310939 Free PMC article. Review.
-
A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development.Cancer Res. 2013 Aug 1;73(15):4810-9. doi: 10.1158/0008-5472.CAN-13-0665. Epub 2013 Jun 19. Cancer Res. 2013. PMID: 23783576 Free PMC article.
-
Bioactive prolactin levels and risk of breast cancer: a nested case-control study.Cancer Epidemiol Biomarkers Prev. 2015 Jan;24(1):73-80. doi: 10.1158/1055-9965.EPI-14-0896. Epub 2014 Oct 14. Cancer Epidemiol Biomarkers Prev. 2015. PMID: 25315962 Free PMC article.
-
SDF-1/CXCR4 signalling is involved in blood vessel growth and remodelling by intussusception.J Cell Mol Med. 2019 Jun;23(6):3916-3926. doi: 10.1111/jcmm.14269. Epub 2019 Apr 4. J Cell Mol Med. 2019. PMID: 30950188 Free PMC article.
References
-
- Horseman ND. Prolactin and mammary gland development. J Mammary Gland Biol Neoplasia. 1999;4:79–88. - PubMed
-
- Bole-Feysot C, Goffin V, Edery M, et al. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–68. - PubMed
-
- Nagano M, Chastre E, Choquet A, et al. Expression of prolactin and growth hormone receptor genes and their isoforms in the gastrointestinal tract. Am J Physiol. 1995;268:G431–42. - PubMed
-
- Peirce SK, Chen WY, Chen WY. Quantification of prolactin receptor mRNA in multiple human tissues and cancer cell lines by real time RT-PCR. J Endocrinol. 2001;171:R1–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

