Palmitoylation influences the function and pharmacology of sodium channels
- PMID: 22123950
- PMCID: PMC3250191
- DOI: 10.1073/pnas.1108497108
Palmitoylation influences the function and pharmacology of sodium channels
Abstract
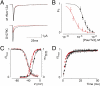
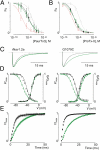
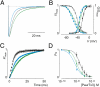
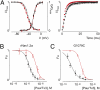
Palmitoylation is a common lipid modification known to regulate the functional properties of various proteins and is a vital step in the biosynthesis of voltage-activated sodium (Nav) channels. We discovered a mutation in an intracellular loop of rNav1.2a (G1079C), which results in a higher apparent affinity for externally applied PaurTx3 and ProTx-II, two voltage sensor toxins isolated from tarantula venom. To explore whether palmitoylation of the introduced cysteine underlies this observation, we compared channel susceptibility to a range of animal toxins in the absence and presence of 2-Br-palmitate, a palmitate analog that prevents palmitate incorporation into proteins, and found that palmitoylation contributes to the increased affinity of PaurTx3 and ProTx-II for G1079C. Further investigations with 2-Br-palmitate revealed that palmitoylation can regulate the gating and pharmacology of wild-type (wt) rNav1.2a. To identify rNav1.2a palmitoylation sites contributing to these phenomena, we substituted three endogenous cysteines predicted to be palmitoylated and found that the gating behavior of this triple cysteine mutant is similar to wt rNav1.2a treated with 2-Br-palmitate. As with chemically depalmitoylated rNav1.2a channels, this mutant also exhibits an increased susceptibility for PaurTx3. Additional mutagenesis experiments showed that palmitoylation of one cysteine in particular (C1182) primarily influences PaurTx3 sensitivity and may enhance the inactivation process of wt rNav1.2a. Overall, our results demonstrate that lipid modifications are capable of altering the gating and pharmacological properties of rNav1.2a.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Inhibition of sodium channel gating by trapping the domain II voltage sensor with protoxin II.Mol Pharmacol. 2008 Mar;73(3):1020-8. doi: 10.1124/mol.107.041046. Epub 2007 Dec 21. Mol Pharmacol. 2008. PMID: 18156314
-
Deglycosylation altered the gating properties of rNav1.3: glycosylation/deglycosylation homeostasis probably complicates the functional regulation of voltage-gated sodium channel.Neurosci Bull. 2008 Oct;24(5):283-7. doi: 10.1007/s12264-008-0524-5. Neurosci Bull. 2008. PMID: 18839021 Free PMC article.
-
Methanethiosulfonate-modification alters local anesthetic block in rNav1.4 cysteine-substituted mutants S1276C and L1280C.J Membr Biol. 2003 May 1;193(1):47-55. doi: 10.1007/s00232-002-2006-4. J Membr Biol. 2003. PMID: 12879165
-
ProTx-I and ProTx-II: gating modifiers of voltage-gated sodium channels.Toxicon. 2007 Feb;49(2):194-201. doi: 10.1016/j.toxicon.2006.09.014. Epub 2006 Sep 27. Toxicon. 2007. PMID: 17087985 Review.
-
Regulation of NCX1 by palmitoylation.Cell Calcium. 2020 Mar;86:102158. doi: 10.1016/j.ceca.2019.102158. Epub 2020 Jan 8. Cell Calcium. 2020. PMID: 31935590 Review.
Cited by
-
Cardiac sodium channel palmitoylation regulates channel availability and myocyte excitability with implications for arrhythmia generation.Nat Commun. 2016 Jun 23;7:12035. doi: 10.1038/ncomms12035. Nat Commun. 2016. PMID: 27337590 Free PMC article.
-
Spider Knottin Pharmacology at Voltage-Gated Sodium Channels and Their Potential to Modulate Pain Pathways.Toxins (Basel). 2019 Oct 29;11(11):626. doi: 10.3390/toxins11110626. Toxins (Basel). 2019. PMID: 31671792 Free PMC article. Review.
-
TRP channels: Role in neurodegenerative diseases and therapeutic targets.Heliyon. 2023 Jun 2;9(6):e16910. doi: 10.1016/j.heliyon.2023.e16910. eCollection 2023 Jun. Heliyon. 2023. PMID: 37332910 Free PMC article. Review.
-
An update on transcriptional and post-translational regulation of brain voltage-gated sodium channels.Amino Acids. 2016 Mar;48(3):641-651. doi: 10.1007/s00726-015-2122-y. Epub 2015 Oct 27. Amino Acids. 2016. PMID: 26503606 Free PMC article. Review.
-
Selective NaV1.1 activation rescues Dravet syndrome mice from seizures and premature death.Proc Natl Acad Sci U S A. 2018 Aug 21;115(34):E8077-E8085. doi: 10.1073/pnas.1804764115. Epub 2018 Aug 3. Proc Natl Acad Sci U S A. 2018. PMID: 30076230 Free PMC article.
References
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. - PubMed
-
- Hille B. Ion Channels of Excitable Membranes. 3rd ed. Sunderland, MA: Sinauer; 2001. p. 814.
-
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. - PubMed
-
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

