HIV-1 protease inhibitors and clinical malaria: a secondary analysis of the AIDS Clinical Trials Group A5208 study
- PMID: 22123685
- PMCID: PMC3264273
- DOI: 10.1128/AAC.05322-11
HIV-1 protease inhibitors and clinical malaria: a secondary analysis of the AIDS Clinical Trials Group A5208 study
Abstract
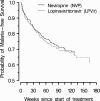
HIV-1 protease inhibitors (PIs) have antimalarial activity in vitro and in murine models. The potential beneficial effect of HIV-1 PIs on malaria has not been studied in clinical settings. We used data from Adult AIDS Clinical Trials Group A5208 sites where malaria is endemic to compare the incidence of clinically diagnosed malaria among HIV-infected adult women randomized to either lopinavir/ritonavir (LPV/r)-based antiretroviral therapy (ART) or to nevirapine (NVP)-based ART. We calculated hazard ratios and 95% confidence intervals. We conducted a recurrent events analysis that included both first and second clinical malarial episodes and also conducted analyses to assess the sensitivity of results to outcome misclassification. Among the 445 women in this analysis, 137 (31%) received a clinical diagnosis of malaria at least once during follow-up. Of these 137, 72 (53%) were randomized to LPV/r-based ART. Assignment to the LPV/r treatment group (n = 226) was not consistent with a large decrease in the hazard of first clinical malarial episode (hazard ratio = 1.11 [0.79 to 1.56]). The results were similar in the recurrent events analysis. Sensitivity analyses indicated the results were robust to reasonable levels of outcome misclassification. In this study, the treatment with LPV/r compared to NVP had no apparent beneficial effect on the incidence of clinical malaria among HIV-infected adult women. Additional research concerning the effects of PI-based therapy on the incidence of malaria diagnosed by more specific criteria and among groups at a higher risk for severe disease is warranted.
Trial registration: ClinicalTrials.gov NCT00089505.
Figures
Similar articles
-
First-line antiretroviral therapy with nevirapine versus lopinavir-ritonavir based regimens in a resource-limited setting.AIDS. 2014 May 15;28(8):1143-53. doi: 10.1097/QAD.0000000000000214. AIDS. 2014. PMID: 25028911 Free PMC article. Clinical Trial.
-
Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/ emtricitabine in antiretroviral-naïve subjects: the progress study, 48-week results.HIV Clin Trials. 2011 Sep-Oct;12(5):255-67. doi: 10.1310/hct1205-255. HIV Clin Trials. 2011. PMID: 22180523 Clinical Trial.
-
Cardiovascular disease risk factors in HIV-infected women after initiation of lopinavir/ritonavir- and nevirapine-based antiretroviral therapy in Sub-Saharan Africa: A5208 (OCTANE).J Acquir Immune Defic Syndr. 2014 Jun 1;66(2):155-63. doi: 10.1097/QAI.0000000000000131. J Acquir Immune Defic Syndr. 2014. PMID: 24562349 Free PMC article. Clinical Trial.
-
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age.Cochrane Database Syst Rev. 2014 May 22;2014(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. Cochrane Database Syst Rev. 2014. PMID: 24852077 Free PMC article. Review.
-
Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD004772. doi: 10.1002/14651858.CD004772.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 May 22;(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. PMID: 22786492 Updated. Review.
Cited by
-
Malaria in HIV-Infected Children Receiving HIV Protease-Inhibitor- Compared with Non-Nucleoside Reverse Transcriptase Inhibitor-Based Antiretroviral Therapy, IMPAACT P1068s, Substudy to P1060.PLoS One. 2016 Dec 9;11(12):e0165140. doi: 10.1371/journal.pone.0165140. eCollection 2016. PLoS One. 2016. PMID: 27936233 Free PMC article. Clinical Trial.
-
Antiretroviral Choice for HIV Impacts Antimalarial Exposure and Treatment Outcomes in Ugandan Children.Clin Infect Dis. 2016 Aug 1;63(3):414-22. doi: 10.1093/cid/ciw291. Epub 2016 May 3. Clin Infect Dis. 2016. PMID: 27143666 Free PMC article.
-
Prevalence of asymptomatic parasitemia and gametocytemia among HIV-infected Ugandan children randomized to receive different antiretroviral therapies.Am J Trop Med Hyg. 2013 Apr;88(4):744-6. doi: 10.4269/ajtmh.12-0658. Epub 2013 Jan 28. Am J Trop Med Hyg. 2013. PMID: 23358639 Free PMC article. Clinical Trial.
-
Artemether-lumefantrine co-administration with antiretrovirals: population pharmacokinetics and dosing implications.Br J Clin Pharmacol. 2015 Apr;79(4):636-49. doi: 10.1111/bcp.12529. Br J Clin Pharmacol. 2015. PMID: 25297720 Free PMC article. Clinical Trial.
-
Presence of Plasmodium falciparum DNA in Plasma Does Not Predict Clinical Malaria in an HIV-1 Infected Population.PLoS One. 2015 Jun 8;10(6):e0129519. doi: 10.1371/journal.pone.0129519. eCollection 2015. PLoS One. 2015. PMID: 26053030 Free PMC article.
References
-
- AIDS Clinical Trials Group 2007. Appendix 60. ACTG/National Institutes of Health, Bethesda, MD
-
- Arrive E, et al. 2007. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int. J. Epidemiol. 36: 1009–1021 - PubMed
-
- Cain LE, et al. 2006. Effect of highly active antiretroviral therapy on multiple AIDS-defining illnesses among male HIV seroconverters. Am. J. Epidemiol. 163: 310–315 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous