Soluble Aβ oligomer production and toxicity
- PMID: 22121920
- PMCID: PMC3254782
- DOI: 10.1111/j.1471-4159.2011.07478.x
Soluble Aβ oligomer production and toxicity
Abstract
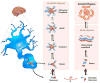
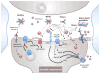
For nearly 100 years following the first description of this neurological disorder by Dr Alois Alzheimer, amyloid plaques and neurofibrillary tangles have been hypothesized to cause neuronal loss. With evidence that the extent of insoluble, deposited amyloid poorly correlated with cognitive impairment, research efforts focused on soluble forms of Aβ, also referred as Aβ oligomers. Following a decade of studies, soluble oligomeric forms of Aβ are now believed to induce the deleterious cascade(s) involved in the pathophysiology of Alzheimer's disease. In this review, we will discuss our current understanding about endogenous oligomeric Aβ production, their relative toxicity in vivo and in vitro, and explore the potential future directions needed for the field.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Conflict of interest statement
Figures



Similar articles
-
[Involvement of beta-amyloid in the etiology of Alzheimer's disease].Brain Nerve. 2010 Jul;62(7):691-9. Brain Nerve. 2010. PMID: 20675873 Review. Japanese.
-
[The pathophysiology of Alzheimer's disease with special reference to "amyloid cascade hypothesis"].Rinsho Byori. 2013 Nov;61(11):1060-9. Rinsho Byori. 2013. PMID: 24450113 Review. Japanese.
-
Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis.JAMA Neurol. 2014 Apr;71(4):505-8. doi: 10.1001/jamaneurol.2013.5847. JAMA Neurol. 2014. PMID: 24493463 Review.
-
Soluble pre-fibrillar tau and β-amyloid species emerge in early human Alzheimer's disease and track disease progression and cognitive decline.Acta Neuropathol. 2016 Dec;132(6):875-895. doi: 10.1007/s00401-016-1632-3. Epub 2016 Oct 21. Acta Neuropathol. 2016. PMID: 27770234 Free PMC article.
-
Oligomeric Abeta in Alzheimer's disease: relationship to plaque and tangle pathology, APOE genotype and cerebral amyloid angiopathy.Brain Pathol. 2010 Mar;20(2):468-80. doi: 10.1111/j.1750-3639.2009.00321.x. Epub 2009 Jul 16. Brain Pathol. 2010. PMID: 19725829 Free PMC article.
Cited by
-
Targeting amyloid proteins for clinical diagnosis of neurodegenerative diseases.Fundam Res. 2022 Nov 1;3(4):505-519. doi: 10.1016/j.fmre.2022.10.009. eCollection 2023 Jul. Fundam Res. 2022. PMID: 38933553 Free PMC article. Review.
-
Early Diagnosis of Neurodegenerative Diseases: What Has Been Undertaken to Promote the Transition from PET to Fluorescence Tracers.Molecules. 2024 Feb 4;29(3):722. doi: 10.3390/molecules29030722. Molecules. 2024. PMID: 38338465 Free PMC article. Review.
-
Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model.J Neurosci. 2013 Mar 13;33(11):5053-64. doi: 10.1523/JNEUROSCI.4361-12.2013. J Neurosci. 2013. PMID: 23486975 Free PMC article.
-
Anesthesia and Cognitive Outcome in Elderly Patients: A Narrative Viewpoint.J Neurosurg Anesthesiol. 2020 Jan;32(1):9-17. doi: 10.1097/ANA.0000000000000640. J Neurosurg Anesthesiol. 2020. PMID: 31490337 Free PMC article. Review.
-
Angiotensin-(1-7) Analogue AVE0991 Modulates Astrocyte-Mediated Neuroinflammation via lncRNA SNHG14/miR-223-3p/NLRP3 Pathway and Offers Neuroprotection in a Transgenic Mouse Model of Alzheimer's Disease.J Inflamm Res. 2021 Dec 18;14:7007-7019. doi: 10.2147/JIR.S343575. eCollection 2021. J Inflamm Res. 2021. PMID: 34955647 Free PMC article.
References
-
- Barghorn S, Nimmrich V, Striebinger A, et al. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005;95:834–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

