An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells
- PMID: 22118527
- PMCID: PMC3431922
- DOI: 10.1016/j.immuni.2011.09.017
An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells
Abstract
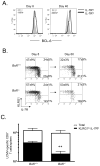
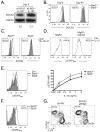
Memory CD8(+) T cells are critical for long-term immunity, but the genetic pathways governing their formation remain poorly defined. This study shows that the IL-10-IL-21-STAT3 pathway is critical for memory CD8(+) T cell development after acute LCMV infection. In the absence of either interleukin-10 (IL-10) and IL-21 or STAT3, virus-specific CD8(+) T cells retain terminal effector (TE) differentiation states and fail to mature into protective memory T cells that contain self-renewing central memory T cells. Expression of Eomes, BCL-6, Blimp-1, and SOCS3 was considerably reduced in STAT3-deficient memory CD8(+) T cells, and BCL-6- or SOCS3-deficient CD8(+) T cells also had perturbed memory cell development. Reduced SOCS3 expression rendered STAT3-deficient CD8(+) T cells hyperresponsive to IL-12, suggesting that the STAT3-SOCS3 pathway helps to insulate memory precursor cells from inflammatory cytokines that drive TE differentiation. Thus, memory CD8(+) T cell precursor maturation is an active process dependent on IL-10-IL-21-STAT3 signaling.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

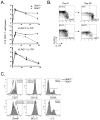
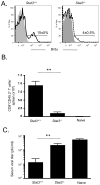
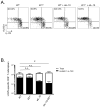
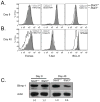
Comment in
-
Keeping STATs on memory CD8+ T cells.Immunity. 2011 Nov 23;35(5):663-5. doi: 10.1016/j.immuni.2011.11.006. Immunity. 2011. PMID: 22118521
Similar articles
-
IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells.Eur J Immunol. 2013 Feb;43(2):468-79. doi: 10.1002/eji.201242930. Epub 2013 Jan 15. Eur J Immunol. 2013. PMID: 23225163 Free PMC article.
-
Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function.J Allergy Clin Immunol. 2013 Aug;132(2):400-11.e9. doi: 10.1016/j.jaci.2013.05.029. Epub 2013 Jul 4. J Allergy Clin Immunol. 2013. PMID: 23830147 Free PMC article.
-
Eomesodermin driven IL-10 production in effector CD8+ T cells promotes a memory phenotype.Cell Immunol. 2019 Jan;335:93-102. doi: 10.1016/j.cellimm.2018.11.008. Epub 2018 Dec 1. Cell Immunol. 2019. PMID: 30528350 Free PMC article.
-
SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis.Semin Immunol. 2014 Dec;26(6):518-32. doi: 10.1016/j.smim.2014.10.004. Epub 2014 Nov 1. Semin Immunol. 2014. PMID: 25458989 Review.
-
Molecular Control of Follicular Helper T cell Development and Differentiation.Front Immunol. 2018 Oct 25;9:2470. doi: 10.3389/fimmu.2018.02470. eCollection 2018. Front Immunol. 2018. PMID: 30410493 Free PMC article. Review.
Cited by
-
The lncRNA Snhg1-Vps13D vesicle trafficking system promotes memory CD8 T cell establishment via regulating the dual effects of IL-7 signaling.Signal Transduct Target Ther. 2021 Mar 24;6(1):126. doi: 10.1038/s41392-021-00492-9. Signal Transduct Target Ther. 2021. PMID: 33758164 Free PMC article.
-
Identification of Gene Regulatory Networks in B-Cell Progenitor Differentiation and Leukemia.Genes (Basel). 2024 Jul 24;15(8):978. doi: 10.3390/genes15080978. Genes (Basel). 2024. PMID: 39202339 Free PMC article.
-
Complexity and Controversies over the Cytokine Profiles of T Helper Cell Subpopulations in Tuberculosis.J Immunol Res. 2015;2015:639107. doi: 10.1155/2015/639107. Epub 2015 Oct 1. J Immunol Res. 2015. PMID: 26495323 Free PMC article. Review.
-
STAT3 Activation-Induced Fatty Acid Oxidation in CD8+ T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth.Cell Metab. 2020 Jan 7;31(1):148-161.e5. doi: 10.1016/j.cmet.2019.10.013. Epub 2019 Nov 21. Cell Metab. 2020. PMID: 31761565 Free PMC article.
-
Mechanisms and consequences of Jak-STAT signaling in the immune system.Nat Immunol. 2017 Mar 22;18(4):374-384. doi: 10.1038/ni.3691. Nat Immunol. 2017. PMID: 28323260 Free PMC article. Review.
References
-
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11:748–756. - PubMed
-
- Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nature immunology. 2004;5:809–817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI074699/AI/NIAID NIH HHS/United States
- R01 AI074699-04/AI/NIAID NIH HHS/United States
- R01AR40072/AR/NIAMS NIH HHS/United States
- R01 AR040072/AR/NIAMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI075157/AI/NIAID NIH HHS/United States
- R21 AI081150/AI/NIAID NIH HHS/United States
- R01 AI066232-07/AI/NIAID NIH HHS/United States
- AR44076/AR/NIAMS NIH HHS/United States
- AI075157/AI/NIAID NIH HHS/United States
- R21AI081150/AI/NIAID NIH HHS/United States
- R01AI066232/AI/NIAID NIH HHS/United States
- R01 AR044076/AR/NIAMS NIH HHS/United States
- R01 AI066232/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

