Polyinosinic acid decreases sequestration and improves systemic therapy of measles virus
- PMID: 22116376
- PMCID: PMC3288770
- DOI: 10.1038/cgt.2011.82
Polyinosinic acid decreases sequestration and improves systemic therapy of measles virus
Abstract
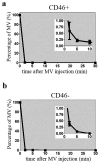
Off-target binding or vector sequestration can significantly limit the efficiency of systemic virotherapy. We report here that systemically administered oncolytic measles virus (MV) was rapidly sequestered by the mononuclear phagocytic system (MPS) of the liver and spleen in measles receptor CD46-positive and CD46-negative mice. Since scavenger receptors on Kupffer cells are responsible for the elimination of blood-borne pathogens, we investigated here if MV uptake was mediated by scavenger receptors on Kupffer cells. Pretreatment of cells with poly(I), a scavenger receptor ligand, reduced MV expression by 99% in murine (J774A.1) macrophages and by 50% in human (THP-1) macrophages. Pre-dosing of mice with poly(I) reduced MPS sequestration of MV and increased circulating levels of MV by 4 to 15-folds at 2 min post virus administration. Circulating virus was still detectable 30 min post infusion in mice pre-dosed with poly(I) whereas no detectable MV was found at 5-10 min post infusion if mice did not receive poly(I). MPS blockade by poly(I) enhanced virus delivery to human ovarian SKOV3ip.1 and myeloma KAS6/1 xenografts in mice. Higher gene expression and improved control of tumor growth was noted early post therapy. Based on these results, incorporation of MPS blockade into MV treatment regimens is warranted.
Figures
Similar articles
-
Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter.Clin Cancer Res. 2006 Mar 15;12(6):1868-75. doi: 10.1158/1078-0432.CCR-05-1803. Clin Cancer Res. 2006. PMID: 16551872
-
Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor.Cancer Res. 2009 Feb 15;69(4):1459-68. doi: 10.1158/0008-5472.CAN-08-2628. Epub 2009 Feb 10. Cancer Res. 2009. PMID: 19208845 Free PMC article.
-
Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model.Clin Cancer Res. 2009 Dec 1;15(23):7246-55. doi: 10.1158/1078-0432.CCR-09-1292. Epub 2009 Nov 24. Clin Cancer Res. 2009. PMID: 19934299 Free PMC article.
-
Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview.Curr Opin Mol Ther. 2009 Feb;11(1):43-53. Curr Opin Mol Ther. 2009. PMID: 19169959 Free PMC article. Review.
-
Measles to the Rescue: A Review of Oncolytic Measles Virus.Viruses. 2016 Oct 22;8(10):294. doi: 10.3390/v8100294. Viruses. 2016. PMID: 27782084 Free PMC article. Review.
Cited by
-
Structure-Activity Relationship of PEGylated Polylysine Peptides as Scavenger Receptor Inhibitors for Non-Viral Gene Delivery.Mol Pharm. 2015 Dec 7;12(12):4321-8. doi: 10.1021/acs.molpharmaceut.5b00513. Epub 2015 Nov 5. Mol Pharm. 2015. PMID: 26485572 Free PMC article.
-
Designing and building oncolytic viruses.Future Virol. 2017 Apr;12(4):193-213. doi: 10.2217/fvl-2016-0129. Epub 2017 Mar 31. Future Virol. 2017. PMID: 29387140 Free PMC article. Review.
-
Measles Virus as an Oncolytic Immunotherapy.Cancers (Basel). 2021 Feb 1;13(3):544. doi: 10.3390/cancers13030544. Cancers (Basel). 2021. PMID: 33535479 Free PMC article. Review.
-
PEG-Peptide Inhibition of Scavenger Receptor Uptake of Nanoparticles by the Liver.Mol Pharm. 2018 Sep 4;15(9):3881-3891. doi: 10.1021/acs.molpharmaceut.8b00355. Epub 2018 Aug 13. Mol Pharm. 2018. PMID: 30052459 Free PMC article.
-
Oncolytic measles virus strains as novel anticancer agents.Expert Opin Biol Ther. 2013 Apr;13(4):483-502. doi: 10.1517/14712598.2013.749851. Epub 2013 Jan 6. Expert Opin Biol Ther. 2013. PMID: 23289598 Free PMC article. Review.
References
-
- Chiocca EA. The host response to cancer virotherapy. Current opinion in molecular therapeutics. 2008;10(1):38–45. - PubMed
-
- Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature clinical practice. 2007;4(2):101–17. - PubMed
-
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. The lancet oncology. 2008;9(6):533–42. - PubMed
-
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA129966/CA/NCI NIH HHS/United States
- CA100634/CA/NCI NIH HHS/United States
- CA125614/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- R01 CA125614/CA/NCI NIH HHS/United States
- CA118488/CA/NCI NIH HHS/United States
- R01 CA100634/CA/NCI NIH HHS/United States
- R01 CA129193/CA/NCI NIH HHS/United States
- R01 CA125614-05/CA/NCI NIH HHS/United States
- R01 CA129966/CA/NCI NIH HHS/United States
- CA129193/CA/NCI NIH HHS/United States
- R01 CA118488/CA/NCI NIH HHS/United States
- N01 CA015083/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

