Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck
- PMID: 22116344
- PMCID: PMC5925419
- DOI: 10.1007/s00262-011-1154-8
Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck
Abstract
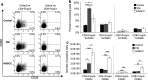
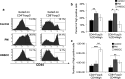
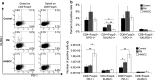
While studies have indicated that squamous cell carcinoma of the head and neck (HNSCC) is associated with immune suppression, these studies did not analyze the immune response at the dysplastic stage. The present study utilized a mouse model of 4-nitroquinoline 1-oxide-induced oral carcinogenesis to examine the alterations in immune phenotype at the premalignant and malignant stages of HNSCC. Cervical lymph nodes of HNSCC-bearing mice were found to contain a greater number of cells, including a greater number of conventional (Tconv) and regulatory (Treg) T cells, compared to cervical lymph nodes of control and premalignant lesion-bearing mice, though the Tconv cells appear to be less proliferative and the Treg cells appear to be less suppressive at the HNSCC stage. Premalignant lesion-bearing mouse lymph nodes consist of a greater percentage of Tconv cells expressing markers for activation, memory, and exhaustion compared to both control and HNSCC-bearing mice. Also, lymph nodes' cells from both premalignant lesion-bearing and HNSCC-bearing mice include increased levels of Th1, Tc1, and Th17 cells, with no differences in levels of Th2 cells, compared to control mice. The data show that while there is the expected increase in immunosuppressive Tregs in lymph nodes when HNSCC is present, there is also an unexpected increase in immune populations usually associated with a beneficial antitumor response, including Tconv cells and Th1 and Tc1 cells. In addition, the results demonstrate that the premalignant stage of HNSCC development is associated with a robust immune response involving an increase in inflammatory Th1, Tc1, and Th17 cells.
Figures



Similar articles
-
Treatment to sustain a Th17-type phenotype to prevent skewing toward Treg and to limit premalignant lesion progression to cancer.Int J Cancer. 2016 May 15;138(10):2487-98. doi: 10.1002/ijc.29989. Epub 2016 Jan 28. Int J Cancer. 2016. PMID: 26756968 Free PMC article.
-
Administration of a vaccine composed of dendritic cells pulsed with premalignant oral lesion lysate to mice bearing carcinogen-induced premalignant oral lesions stimulates a protective immune response.Int Immunopharmacol. 2012 Jul;13(3):322-30. doi: 10.1016/j.intimp.2012.05.004. Epub 2012 May 16. Int Immunopharmacol. 2012. PMID: 22609090 Free PMC article.
-
Black raspberry extract inhibits regulatory T-cell activity in a murine model of head and neck squamous cell carcinoma chemoprevention.Front Immunol. 2022 Aug 9;13:932742. doi: 10.3389/fimmu.2022.932742. eCollection 2022. Front Immunol. 2022. PMID: 36016924 Free PMC article.
-
Cytokines in head and neck cancer.Cytokine Growth Factor Rev. 2006 Jun;17(3):141-6. doi: 10.1016/j.cytogfr.2006.02.001. Epub 2006 Mar 15. Cytokine Growth Factor Rev. 2006. PMID: 16540364 Review.
-
Regulatory T cells: what role do they play in antitumor immunity in patients with head and neck cancer?Head Neck. 2008 Feb;30(2):251-61. doi: 10.1002/hed.20739. Head Neck. 2008. PMID: 18172882 Review.
Cited by
-
Simultaneous Expression of Th1- and Treg-Associated Chemokine Genes and CD4+, CD8+, and Foxp3+ Cells in the Premalignant Lesions of 4NQO-Induced Mouse Tongue Tumorigenesis.Cancers (Basel). 2021 Apr 12;13(8):1835. doi: 10.3390/cancers13081835. Cancers (Basel). 2021. PMID: 33921389 Free PMC article.
-
Adipocytes as immune regulatory cells.Int Immunopharmacol. 2013 Jun;16(2):224-31. doi: 10.1016/j.intimp.2013.04.002. Epub 2013 Apr 13. Int Immunopharmacol. 2013. PMID: 23587489 Free PMC article.
-
Effect of the premalignant and tumor microenvironment on immune cell cytokine production in head and neck cancer.Cancers (Basel). 2014 Apr 2;6(2):756-70. doi: 10.3390/cancers6020756. Cancers (Basel). 2014. PMID: 24698959 Free PMC article.
-
An Inflammatory Cytokine Milieu is Prominent in Premalignant Oral Lesions, but Subsides when Lesions Progress to Squamous Cell Carcinoma.J Clin Cell Immunol. 2014 Jun;5(3):230. doi: 10.4172/2155-9899.1000230. J Clin Cell Immunol. 2014. PMID: 25419481 Free PMC article.
-
Anti-Tumor Immunity in Head and Neck Cancer: Understanding the Evidence, How Tumors Escape and Immunotherapeutic Approaches.Cancers (Basel). 2015 Dec 9;7(4):2397-414. doi: 10.3390/cancers7040900. Cancers (Basel). 2015. PMID: 26690220 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

