Plasmodium falciparum possesses a unique dual-specificity serine/threonine and tyrosine kinase, Pfnek3
- PMID: 22116321
- PMCID: PMC11114921
- DOI: 10.1007/s00018-011-0888-y
Plasmodium falciparum possesses a unique dual-specificity serine/threonine and tyrosine kinase, Pfnek3
Abstract
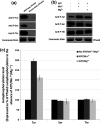
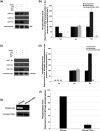
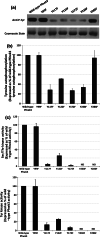
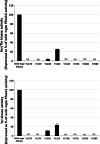
Despite the absence of classical tyrosine kinases encrypted in the kinome of Plasmodium falciparum, biochemical analyses have detected significant tyrosine phosphorylation in its cell lysates. Supporting such phosphorylation is critical for parasite development. These observations have thus raised queries regarding the plasmodial enzymes accountable for tyrosine kinase activities in vivo. In the current investigation, immunoblot analysis intriguingly demonstrated that Pfnek3, a plasmodial mitogen-activated protein kinase kinase (MAPKK), displayed both serine/threonine and tyrosine kinase activities in autophosphorylation reactions as well as in phosphorylation of the exogenous myelin basic protein substrate. The results obtained strongly support Pfnek3 as a novel dual-specificity kinase of the malarial parasite, even though it displays a HGDLKSTN motif in the catalytic loop that resembles the consensus HRDLKxxN signature found in the serine/threonine kinases. Notably, its serine/threonine and tyrosine kinase activities were found to be distinctly influenced by Mg(2+) and Mn(2+) cofactors. Further probing into the regulatory mechanism of Pfnek3 also revealed tyrosine phosphorylation to be a crucial factor that stimulates its kinase activity. Through biocomputational analyses and functional assays, tyrosine residues Y117, Y122, Y172, and Y238 were proposed as phosphorylation sites essential for mediating the catalytic activities of Pfnek3. The discovery of Pfnek3's dual role in phosphorylation marks its importance in closing the loop for cellular regulation in P. falciparum, which remains elusive to date.
Figures


Similar articles
-
Regulation of Plasmodium falciparum Pfnek3 relies on phosphorylation at its activation loop and at threonine 82.Cell Mol Life Sci. 2009 Sep;66(18):3081-90. doi: 10.1007/s00018-009-0101-8. Epub 2009 Jul 31. Cell Mol Life Sci. 2009. PMID: 19644735 Free PMC article.
-
Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum Biochemical properties and possible involvement in MAPK regulation.Eur J Biochem. 2001 May;268(9):2600-8. doi: 10.1046/j.1432-1327.2001.02151.x. Eur J Biochem. 2001. PMID: 11322879
-
Pfnek3 functions as an atypical MAPKK in Plasmodium falciparum.Biochem Biophys Res Commun. 2007 Sep 21;361(2):439-44. doi: 10.1016/j.bbrc.2007.07.047. Epub 2007 Jul 20. Biochem Biophys Res Commun. 2007. PMID: 17662247
-
Pfnek3: an atypical activator of a MAP kinase in Plasmodium falciparum.FEBS Lett. 2006 Nov 13;580(26):6083-92. doi: 10.1016/j.febslet.2006.10.003. Epub 2006 Oct 12. FEBS Lett. 2006. PMID: 17064692
-
TKL family kinases in human apicomplexan pathogens.Mol Biochem Parasitol. 2024 Sep;259:111628. doi: 10.1016/j.molbiopara.2024.111628. Epub 2024 May 6. Mol Biochem Parasitol. 2024. PMID: 38719028 Review.
Cited by
-
Inhibition of an Erythrocyte Tyrosine Kinase with Imatinib Prevents Plasmodium falciparum Egress and Terminates Parasitemia.PLoS One. 2016 Oct 21;11(10):e0164895. doi: 10.1371/journal.pone.0164895. eCollection 2016. PLoS One. 2016. PMID: 27768734 Free PMC article.
-
Malaria protein kinase CK2 (PfCK2) shows novel mechanisms of regulation.PLoS One. 2014 Mar 21;9(3):e85391. doi: 10.1371/journal.pone.0085391. eCollection 2014. PLoS One. 2014. PMID: 24658579 Free PMC article.
-
Unveiling the novel dual specificity protein kinases in Bacillus anthracis: identification of the first prokaryotic dual specificity tyrosine phosphorylation-regulated kinase (DYRK)-like kinase.J Biol Chem. 2012 Aug 3;287(32):26749-63. doi: 10.1074/jbc.M112.351304. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711536 Free PMC article.
-
Human Polo-like Kinase Inhibitors as Antiplasmodials.ACS Infect Dis. 2023 Apr 14;9(4):1004-1021. doi: 10.1021/acsinfecdis.3c00025. Epub 2023 Mar 15. ACS Infect Dis. 2023. PMID: 36919909 Free PMC article.
-
Global analysis of protein expression and phosphorylation of three stages of Plasmodium falciparum intraerythrocytic development.J Proteome Res. 2013 Sep 6;12(9):4028-45. doi: 10.1021/pr400394g. Epub 2013 Aug 26. J Proteome Res. 2013. PMID: 23914800 Free PMC article.
References
-
- Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9(8):576–596. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

