Molecular pathogenesis of secondary acute promyelocytic leukemia
- PMID: 22110895
- PMCID: PMC3219647
- DOI: 10.4084/MJHID.2011.045
Molecular pathogenesis of secondary acute promyelocytic leukemia
Abstract
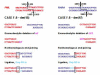
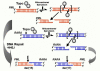
Balanced chromosomal translocations that generate chimeric oncoproteins are considered to be initiating lesions in the pathogenesis of acute myeloid leukemia. The most frequent is the t(15;17)(q22;q21), which fuses the PML and RARA genes, giving rise to acute promyelocytic leukemia (APL). An increasing proportion of APL cases are therapy-related (t-APL), which develop following exposure to radiotherapy and/or chemotherapeutic agents that target DNA topoisomerase II (topoII), particularly mitoxantrone and epirubicin. To gain insights into molecular mechanisms underlying the formation of the t(15;17) we mapped the translocation breakpoints in a series of t-APLs, which revealed significant clustering according to the nature of the drug exposure. Remarkably, in approximately half of t-APL cases arising following mitoxantrone treatment for breast cancer or multiple sclerosis, the chromosome 15 breakpoint fell within an 8-bp "hotspot" region in PML intron 6, which was confirmed to be a preferential site of topoII-mediated DNA cleavage induced by mitoxantrone. Chromosome 15 breakpoints falling outside the "hotspot", and the corresponding RARA breakpoints were also shown to be functional topoII cleavage sites. The observation that particular regions of the PML and RARA loci are susceptible to topoII-mediated DNA damage induced by epirubicin and mitoxantrone may underlie the propensity of these agents to cause APL.
Figures

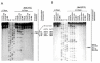
Similar articles
-
Evidence for direct involvement of epirubicin in the formation of chromosomal translocations in t(15;17) therapy-related acute promyelocytic leukemia.Blood. 2010 Jan 14;115(2):326-30. doi: 10.1182/blood-2009-07-235051. Epub 2009 Nov 2. Blood. 2010. PMID: 19884644 Free PMC article.
-
Molecular analysis of t(15;17) genomic breakpoints in secondary acute promyelocytic leukemia arising after treatment of multiple sclerosis.Blood. 2008 Oct 15;112(8):3383-90. doi: 10.1182/blood-2007-10-115600. Epub 2008 Jul 23. Blood. 2008. PMID: 18650449 Free PMC article.
-
Analysis of t(15;17) chromosomal breakpoint sequences in therapy-related versus de novo acute promyelocytic leukemia: association of DNA breaks with specific DNA motifs at PML and RARA loci.Genes Chromosomes Cancer. 2010 Aug;49(8):726-32. doi: 10.1002/gcc.20783. Genes Chromosomes Cancer. 2010. PMID: 20544846
-
Variant and masked translocations in acute promyelocytic leukemia.Leuk Lymphoma. 1996 Jul;22(3-4):221-8. doi: 10.3109/10428199609051752. Leuk Lymphoma. 1996. PMID: 8819070 Review.
-
Novel treatment of acute promyelocytic leukemia: As₂O₃, retinoic acid and retinoid pharmacology.Curr Pharm Biotechnol. 2013;14(9):849-58. doi: 10.2174/1389201015666140113095812. Curr Pharm Biotechnol. 2013. PMID: 24433507 Review.
Cited by
-
Novel, Potent, and Druglike Tetrahydroquinazoline Inhibitor That Is Highly Selective for Human Topoisomerase II α over β.J Med Chem. 2020 Nov 12;63(21):12873-12886. doi: 10.1021/acs.jmedchem.0c00774. Epub 2020 Oct 20. J Med Chem. 2020. PMID: 33079544 Free PMC article.
-
Therapy-related acute promyelocytic leukemia with FMS-like tyrosine kinase 3-internal tandem duplication mutation in solitary bone plasmacytoma: A case report.World J Clin Cases. 2020 Oct 6;8(19):4579-4587. doi: 10.12998/wjcc.v8.i19.4579. World J Clin Cases. 2020. PMID: 33083421 Free PMC article.
-
Concentration-response studies of the chromosome-damaging effects of topoisomerase II inhibitors determined in vitro using human TK6 cells.Mutat Res Genet Toxicol Environ Mutagen. 2019 May;841:49-56. doi: 10.1016/j.mrgentox.2019.05.006. Epub 2019 May 15. Mutat Res Genet Toxicol Environ Mutagen. 2019. PMID: 31138411 Free PMC article.
-
Topoisomerase II and leukemia.Ann N Y Acad Sci. 2014 Mar;1310(1):98-110. doi: 10.1111/nyas.12358. Epub 2014 Feb 3. Ann N Y Acad Sci. 2014. PMID: 24495080 Free PMC article. Review.
-
Topoisomerase-mediated chromosomal break repair: an emerging player in many games.Nat Rev Cancer. 2015 Mar;15(3):137-51. doi: 10.1038/nrc3892. Epub 2015 Feb 19. Nat Rev Cancer. 2015. PMID: 25693836 Review.
References
-
- Mitelman F, Johansson B, Mertens F, editors. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. 2011. http://cgap.nci.nih.gov/Chromosomes/Mitelman.

