A novel pathway for human endothelial cell activation by antiphospholipid/anti-β2 glycoprotein I antibodies
- PMID: 22106343
- PMCID: PMC3265208
- DOI: 10.1182/blood-2011-03-344671
A novel pathway for human endothelial cell activation by antiphospholipid/anti-β2 glycoprotein I antibodies
Abstract
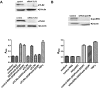
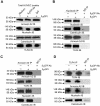
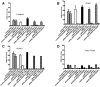
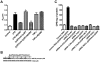
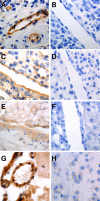
Antiphospholipid Abs (APLAs) are associated with thrombosis and recurrent fetal loss. These Abs are primarily directed against phospholipid-binding proteins, particularly β(2)GPI, and activate endothelial cells (ECs) in a β(2)GPI-dependent manner after binding of β(2)GPI to EC annexin A2. Because annexin A2 is not a transmembrane protein, the mechanisms of APLA/anti-β(2)GPI Ab-mediated EC activation are uncertain, although a role for a TLR4/myeloid differentiation factor 88-dependent pathway leading to activation of NF-κB has been proposed. In the present study, we confirm a critical role for TLR4 in anti-β(2)GPI Ab-mediated EC activation and demonstrate that signaling through TLR4 is mediated through the assembly of a multiprotein signaling complex on the EC surface that includes annexin A2, TLR4, calreticulin, and nucleolin. An essential role for each of these proteins in cell activation is suggested by the fact that inhibiting the expression of each using specific siRNAs blocked EC activation mediated by APLAs/anti-β(2)GPI Abs. These results provide new evidence for novel protein-protein interactions on ECs that may contribute to EC activation and the pathogenesis of APLA/anti-β(2)GPI-associated thrombosis and suggest potential new targets for therapeutic intervention in antiphospholipid syndrome.
Figures
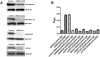
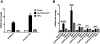
Similar articles
-
A novel pathway of cellular activation mediated by antiphospholipid antibody-induced extracellular vesicles.J Thromb Haemost. 2015 Oct;13(10):1928-40. doi: 10.1111/jth.13072. Epub 2015 Sep 15. J Thromb Haemost. 2015. PMID: 26264622 Free PMC article.
-
Annexin A2: biology and relevance to the antiphospholipid syndrome.Lupus. 2008 Oct;17(10):943-51. doi: 10.1177/0961203308095329. Lupus. 2008. PMID: 18827060 Free PMC article. Review.
-
Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts.Arthritis Rheum. 2007 Aug;56(8):2687-97. doi: 10.1002/art.22802. Arthritis Rheum. 2007. PMID: 17665396
-
Anti-β(2)GPI/β(2)GPI induced TF and TNF-α expression in monocytes involving both TLR4/MyD88 and TLR4/TRIF signaling pathways.Mol Immunol. 2013 Mar;53(3):246-54. doi: 10.1016/j.molimm.2012.08.012. Epub 2012 Sep 8. Mol Immunol. 2013. PMID: 22964479
-
The role of TLR4 in pathophysiology of antiphospholipid syndrome-associated thrombosis and pregnancy morbidity.Br J Haematol. 2014 Jan;164(2):165-76. doi: 10.1111/bjh.12587. Epub 2013 Oct 8. Br J Haematol. 2014. PMID: 24180619 Review.
Cited by
-
Interaction of antiphospholipid antibodies with endothelial cells in antiphospholipid syndrome.Front Immunol. 2024 Jul 9;15:1361519. doi: 10.3389/fimmu.2024.1361519. eCollection 2024. Front Immunol. 2024. PMID: 39044818 Free PMC article. Review.
-
Molecular Insights on the Possible Role of Annexin A2 in COVID-19 Pathogenesis and Post-Infection Complications.Int J Mol Sci. 2021 Oct 13;22(20):11028. doi: 10.3390/ijms222011028. Int J Mol Sci. 2021. PMID: 34681689 Free PMC article. Review.
-
Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target.JCI Insight. 2017 Sep 21;2(18):e93897. doi: 10.1172/jci.insight.93897. eCollection 2017 Sep 21. JCI Insight. 2017. PMID: 28931754 Free PMC article.
-
Nucleolin acts as the receptor for C1QTNF4 and supports C1QTNF4-mediated innate immunity modulation.J Biol Chem. 2021 Jan-Jun;296:100513. doi: 10.1016/j.jbc.2021.100513. Epub 2021 Mar 4. J Biol Chem. 2021. PMID: 33676896 Free PMC article.
-
Endothelial Cell-Activating Antibodies in COVID-19.Arthritis Rheumatol. 2022 Jul;74(7):1132-1138. doi: 10.1002/art.42094. Epub 2022 May 27. Arthritis Rheumatol. 2022. PMID: 35174669 Free PMC article.
References
-
- Rand JH. The antiphospholipid syndrome. Ann Rev Med. 2003;54:409–424. - PubMed
-
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498–1509. - PubMed
-
- Pierangeli SS, Chen PP, Raschi E, et al. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008;34(3):236–250. - PubMed
-
- Cervera R, Khamashta MA, Shoenfeld Y, et al. Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2009;68:1428–1432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

