Interspecies communication in the gut, from bacterial delivery to host-cell response
- PMID: 22106176
- PMCID: PMC3379691
- DOI: 10.1113/jphysiol.2011.220822
Interspecies communication in the gut, from bacterial delivery to host-cell response
Abstract
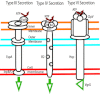
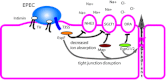
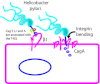
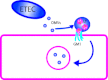
Intestinal pathogens have a wide variety of strategies for communicating with host epithelial cells. This review highlights a few key examples of those strategies. Enteropathogenic Escherichia coli (EPEC) use a type III secretion system (T3SS) to alter host ion transport through both transcriptional and post-translational mechanisms. Salmonella use a similar T3SS to invade host cells and modify an intracellular vacuole, which also impacts host vesicle trafficking. Helicobacter pylori use host cell integrins to provide a conformational change which drives the type IV secretion system into the host cell for delivery of CagA. The novel type VI section systems are phage-like apparati that deliver VgrG-1, which causes actin cross-linking and fluid accumulation in a suckling mouse model. An entirely different delivery mechanism is the outer membrane vesicle (OMV) which is composed of bacterial outer membrane wrapped around contents of the periplamsic space. Enterotoxigenic E. coli use OMVs to deliver bundles of heat labile enterotoxin to host cells. Finally we discuss the host responses to these varied methods of communication.
Figures

Similar articles
-
All in-Multiple parallel strategies for intracellular delivery by bacterial pathogens.Int J Med Microbiol. 2018 Oct;308(7):872-881. doi: 10.1016/j.ijmm.2018.06.007. Epub 2018 Jun 19. Int J Med Microbiol. 2018. PMID: 29936031 Review.
-
EspFu-Mediated Actin Assembly Enhances Enteropathogenic Escherichia coli Adherence and Activates Host Cell Inflammatory Signaling Pathways.mBio. 2020 Apr 14;11(2):e00617-20. doi: 10.1128/mBio.00617-20. mBio. 2020. PMID: 32291304 Free PMC article.
-
Type VI secretion and anti-host effectors.Curr Opin Microbiol. 2016 Feb;29:81-93. doi: 10.1016/j.mib.2015.11.006. Epub 2015 Dec 24. Curr Opin Microbiol. 2016. PMID: 26722980 Review.
-
Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells.Infect Immun. 2012 Dec;80(12):4089-98. doi: 10.1128/IAI.00161-12. Epub 2012 Sep 10. Infect Immun. 2012. PMID: 22966047 Free PMC article.
-
Dual infection system identifies a crucial role for PKA-mediated serine phosphorylation of the EPEC-Tir-injected effector protein in regulating Rac1 function.Cell Microbiol. 2009 Aug;11(8):1254-71. doi: 10.1111/j.1462-5822.2009.01330.x. Epub 2009 Apr 30. Cell Microbiol. 2009. PMID: 19438518
Cited by
-
In vitro coculture assay to assess pathogen induced neutrophil trans-epithelial migration.J Vis Exp. 2014 Jan 6;(83):e50823. doi: 10.3791/50823. J Vis Exp. 2014. PMID: 24430378 Free PMC article.
-
Valid Presumption of Shiga Toxin-Mediated Damage of Developing Erythrocytes in EHEC-Associated Hemolytic Uremic Syndrome.Toxins (Basel). 2020 Jun 4;12(6):373. doi: 10.3390/toxins12060373. Toxins (Basel). 2020. PMID: 32512916 Free PMC article. Review.
-
Sepsis-Like Systemic Inflammation Induced by Nano-Sized Extracellular Vesicles From Feces.Front Microbiol. 2018 Aug 7;9:1735. doi: 10.3389/fmicb.2018.01735. eCollection 2018. Front Microbiol. 2018. PMID: 30131776 Free PMC article.
-
Anti-infective activities of lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents.Clin Microbiol Rev. 2014 Apr;27(2):167-99. doi: 10.1128/CMR.00080-13. Clin Microbiol Rev. 2014. PMID: 24696432 Free PMC article. Review.
-
Effect of Hcp Iron Ion Regulation on the Interaction Between Acinetobacter baumannii With Human Pulmonary Alveolar Epithelial Cells and Biofilm Formation.Front Cell Infect Microbiol. 2022 Feb 23;12:761604. doi: 10.3389/fcimb.2022.761604. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35281445 Free PMC article.
References
-
- Abrahams GL, Muller P, Hensel M. Functional dissection of SseF, a type III effector protein involved in positioning the Salmonella-containing vacuole. Traffic. 2006;7:950–965. - PubMed
-
- Abreu MT, Thomas LS, Arnold ET, Lukasek K, Michelsen KS, Arditi M. TLRsignalling at the intestinal epithelial interface. J Endotoxin Res. 2003;9:322–330. - PubMed
-
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. - PubMed
-
- Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15:163–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

