IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus
- PMID: 22088620
- PMCID: PMC3307526
- DOI: 10.1136/annrheumdis-2011-200463
IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus
Abstract
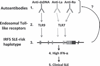
Objective: High serum interferon α (IFNα) activity is a heritable risk factor for systemic lupus erythematosus (SLE). Auto-antibodies found in SLE form immune complexes which can stimulate IFNα production by activating endosomal Toll-like receptors and interferon regulatory factors (IRFs), including IRF5. Genetic variation in IRF5 is associated with SLE susceptibility; however, it is unclear how IRF5 functional genetic elements contribute to human disease.
Methods: 1034 patients with SLE and 989 controls of European ancestry, 555 patients with SLE and 679 controls of African-American ancestry, and 73 patients with SLE of South African ancestry were genotyped at IRF5 polymorphisms, which define major haplotypes. Serum IFNα activity was measured using a functional assay.
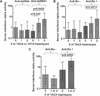
Results: In European ancestry subjects, anti-double-stranded DNA (dsDNA) and anti-Ro antibodies were each associated with different haplotypes characterised by a different combination of functional genetic elements (OR>2.56, p<1.9×10(-14) for both). These IRF5 haplotype-auto-antibody associations strongly predicted higher serum IFNα in patients with SLE and explained >70% of the genetic risk of SLE due to IRF5. In African-American patients with SLE a similar relationship between serology and IFNα was observed, although the previously described European ancestry-risk haplotype was present at admixture proportions in African-American subjects and absent in African patients with SLE.
Conclusions: The authors define a novel risk haplotype of IRF5 that is associated with anti-dsDNA antibodies and show that risk of SLE due to IRF5 genotype is largely dependent upon particular auto-antibodies. This suggests that auto-antibodies are directly pathogenic in human SLE, resulting in increased IFNα in cooperation with particular combinations of IRF5 functional genetic elements. SLE is a systemic autoimmune disorder affecting multiple organ systems including the skin, musculoskeletal, renal and haematopoietic systems. Humoral autoimmunity is a hallmark of SLE, and patients frequently have circulating auto-antibodies directed against dsDNA, as well as RNA binding proteins (RBP). Anti-RBP autoantibodies include antibodies which recognize Ro, La, Smith (anti-Sm), and ribonucleoprotein (anti-nRNP), collectively referred to as anti-retinol-binding protein). Anti-retinol-binding protein and anti-dsDNA auto-antibodies are rare in the healthy population. These auto-antibodies can be present in sera for years preceding the onset of clinical SLE illness and are likely pathogenic in SLE.
Figures

Similar articles
-
Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients.Arthritis Rheum. 2008 Aug;58(8):2481-7. doi: 10.1002/art.23613. Arthritis Rheum. 2008. PMID: 18668568 Free PMC article.
-
Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients.Arthritis Rheum. 2010 Feb;62(2):553-61. doi: 10.1002/art.27182. Arthritis Rheum. 2010. PMID: 20112359 Free PMC article.
-
Brief Report: IRF5 systemic lupus erythematosus risk haplotype is associated with asymptomatic serologic autoimmunity and progression to clinical autoimmunity in mothers of children with neonatal lupus.Arthritis Rheum. 2012 Oct;64(10):3383-7. doi: 10.1002/art.34571. Arthritis Rheum. 2012. PMID: 22674082 Free PMC article.
-
Interferon regulatory factors in human lupus pathogenesis.Transl Res. 2011 Jun;157(6):326-31. doi: 10.1016/j.trsl.2011.01.006. Epub 2011 Feb 8. Transl Res. 2011. PMID: 21575916 Free PMC article. Review.
-
Coordination between innate immune cells, type I IFNs and IRF5 drives SLE pathogenesis.Cytokine. 2020 Aug;132:154731. doi: 10.1016/j.cyto.2019.05.018. Epub 2019 May 23. Cytokine. 2020. PMID: 31130331 Review.
Cited by
-
Genetic and chemical inhibition of IRF5 suppresses pre-existing mouse lupus-like disease.Nat Commun. 2021 Jul 19;12(1):4379. doi: 10.1038/s41467-021-24609-4. Nat Commun. 2021. PMID: 34282144 Free PMC article.
-
Defining biological subsets in systemic lupus erythematosus: progress toward personalized therapy.Pharmaceut Med. 2017 Apr;31(2):81-88. doi: 10.1007/s40290-017-0178-6. Epub 2017 Jan 25. Pharmaceut Med. 2017. PMID: 28827978 Free PMC article.
-
Emerging concepts of type I interferons in SLE pathogenesis and therapy.Nat Rev Rheumatol. 2022 Oct;18(10):575-590. doi: 10.1038/s41584-022-00826-z. Epub 2022 Sep 12. Nat Rev Rheumatol. 2022. PMID: 36097207 Review.
-
Type I Interferons in Systemic Autoimmune Diseases: Distinguishing Between Afferent and Efferent Functions for Precision Medicine and Individualized Treatment.Front Pharmacol. 2021 Apr 14;12:633821. doi: 10.3389/fphar.2021.633821. eCollection 2021. Front Pharmacol. 2021. PMID: 33986670 Free PMC article. Review.
-
Genetic Versus Non-genetic Drivers of SLE: Implications of IRF5 Dysregulation in Both Roads Leading to SLE.Curr Rheumatol Rep. 2019 Jan 15;21(1):2. doi: 10.1007/s11926-019-0803-3. Curr Rheumatol Rep. 2019. PMID: 30645688 Review.
References
-
- Ramos PS, Kelly JA, Gray-McGuire C, et al. Familial aggregation and linkage analysis of autoantibody traits in pedigrees multiplex for systemic lupus erythematosus. Genes Immun. 2006;7:417–432. - PubMed
-
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. - PubMed
-
- Lövgren T, Eloranta ML, Båve U, et al. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. - PubMed
-
- Koffler D, Agnello V, Carr RI, et al. Anti-DNA antibodies and renal lesions of patients with systemic lupus erythematosus. Transplant Proc. 1969;1:933–938. - PubMed
-
- Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–23390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR042476/AR/NIAMS NIH HHS/United States
- R56 AI063274/AI/NIAID NIH HHS/United States
- AI62629/AI/NIAID NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- AR058621/AR/NIAMS NIH HHS/United States
- P50 AR048940/AR/NIAMS NIH HHS/United States
- AR045084/AR/NIAMS NIH HHS/United States
- DE15223/DE/NIDCR NIH HHS/United States
- R01 DE015223/DE/NIDCR NIH HHS/United States
- RR024999/RR/NCRR NIH HHS/United States
- N01AR62277/AR/NIAMS NIH HHS/United States
- L30 AI071651-04/AI/NIAID NIH HHS/United States
- R01 AI063274/AI/NIAID NIH HHS/United States
- AR49084/AR/NIAMS NIH HHS/United States
- AI083790/AI/NIAID NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- AI31584/AI/NIAID NIH HHS/United States
- P20 RR020143/RR/NCRR NIH HHS/United States
- P30 GM103510/GM/NIGMS NIH HHS/United States
- K08 AI083790-03/AI/NIAID NIH HHS/United States
- AR42460/AR/NIAMS NIH HHS/United States
- RR15577/RR/NCRR NIH HHS/United States
- UL1 RR024999/RR/NCRR NIH HHS/United States
- P30 AR053483/AR/NIAMS NIH HHS/United States
- RR20143/RR/NCRR NIH HHS/United States
- R01 AI031584/AI/NIAID NIH HHS/United States
- R01 AR060861-01/AR/NIAMS NIH HHS/United States
- CA141700/CA/NCI NIH HHS/United States
- K08 AI083790/AI/NIAID NIH HHS/United States
- MO1-RR0048/RR/NCRR NIH HHS/United States
- AR060861/AR/NIAMS NIH HHS/United States
- P20 RR015577/RR/NCRR NIH HHS/United States
- P30 RR031152/RR/NCRR NIH HHS/United States
- AR053483/AR/NIAMS NIH HHS/United States
- AI24717/AI/NIAID NIH HHS/United States
- AR052125/AR/NIAMS NIH HHS/United States
- M01 RR000048/RR/NCRR NIH HHS/United States
- MO1-RR00052/RR/NCRR NIH HHS/United States
- RR025741/RR/NCRR NIH HHS/United States
- R01 AR052125/AR/NIAMS NIH HHS/United States
- AI063274/AI/NIAID NIH HHS/United States
- RC1 AR058621/AR/NIAMS NIH HHS/United States
- DK42086/DK/NIDDK NIH HHS/United States
- RR-01070/RR/NCRR NIH HHS/United States
- AI071651/AI/NIAID NIH HHS/United States
- U19 AI062629/AI/NIAID NIH HHS/United States
- UL1 TR000062/TR/NCATS NIH HHS/United States
- P60 AR049459/AR/NIAMS NIH HHS/United States
- R01 AI059893/AI/NIAID NIH HHS/United States
- R37 AI024717/AI/NIAID NIH HHS/United States
- R01 AR042460/AR/NIAMS NIH HHS/United States
- R01 AR033062/AR/NIAMS NIH HHS/United States
- AI065687/AI/NIAID NIH HHS/United States
- R01 AI024717/AI/NIAID NIH HHS/United States
- AR33062,/AR/NIAMS NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- UL1 RR029882/RR/NCRR NIH HHS/United States
- L30 AI071651/AI/NIAID NIH HHS/United States
- R01 CA141700/CA/NCI NIH HHS/United States
- P01 AI065687/AI/NIAID NIH HHS/United States
- K24 AR002138/AR/NIAMS NIH HHS/United States
- AR42476/AR/NIAMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- M01 RR001070/RR/NCRR NIH HHS/United States
- AI53747/AI/NIAID NIH HHS/United States
- AI059893/AI/NIAID NIH HHS/United States
- R01 AR060861/AR/NIAMS NIH HHS/United States
- P60 AR030692/AR/NIAMS NIH HHS/United States
- R21 AI053747/AI/NIAID NIH HHS/United States
- AR48940/AR/NIAMS NIH HHS/United States
- P01 AR049084/AR/NIAMS NIH HHS/United States
- AR002138/AR/NIAMS NIH HHS/United States
- AR049459/AR/NIAMS NIH HHS/United States
- UL1RR029882/RR/NCRR NIH HHS/United States
- P60 AR062755/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
