Visualizing cell death in experimental focal cerebral ischemia: promises, problems, and perspectives
- PMID: 22086195
- PMCID: PMC3272608
- DOI: 10.1038/jcbfm.2011.150
Visualizing cell death in experimental focal cerebral ischemia: promises, problems, and perspectives
Abstract
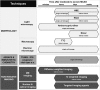
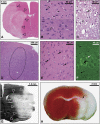
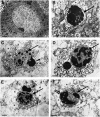
One of the hallmarks of stroke pathophysiology is the widespread death of many different types of brain cells. As our understanding of the complex disease that is stroke has grown, it is now generally accepted that various different mechanisms can result in cell damage and eventual death. A plethora of techniques is available to identify various pathological features of cell death in stroke; each has its own drawbacks and pitfalls, and most are unable to distinguish between different types of cell death, which partially explains the widespread misuse of many terms. The purpose of this review is to summarize the standard histopathological and immunohistochemical techniques used to identify various pathological features of stroke. We then discuss how these methods should be properly interpreted on the basis of what they are showing, as well as advantages and disadvantages that require consideration. As there is much interest in the visualization of stroke using noninvasive imaging strategies, we also specifically discuss how these techniques can be interpreted within the context of cell death.
Figures

Similar articles
-
Present status and future challenges of electroencephalography- and magnetic resonance imaging-based monitoring in preclinical models of focal cerebral ischemia.Brain Res Bull. 2014 Mar;102:22-36. doi: 10.1016/j.brainresbull.2014.01.003. Epub 2014 Jan 23. Brain Res Bull. 2014. PMID: 24462642 Review.
-
Mitochondrial contributions to tissue damage in stroke.Neurochem Int. 2002 May;40(6):511-26. doi: 10.1016/s0197-0186(01)00122-x. Neurochem Int. 2002. PMID: 11850108 Review.
-
A novel multi-modal platform to image molecular and elemental alterations in ischemic stroke.Neurobiol Dis. 2016 Jul;91:132-42. doi: 10.1016/j.nbd.2016.03.006. Epub 2016 Mar 9. Neurobiol Dis. 2016. PMID: 26969531
-
Pathophysiologic cascades in ischemic stroke.Int J Stroke. 2012 Jul;7(5):378-85. doi: 10.1111/j.1747-4949.2012.00839.x. Int J Stroke. 2012. PMID: 22712739 Free PMC article. Review.
-
Imaging of the brain and cerebral vasculature in patients with suspected stroke: advantages and disadvantages of CT and MRI.Curr Neurol Neurosci Rep. 2006 Jan;6(1):9-16. doi: 10.1007/s11910-996-0003-1. Curr Neurol Neurosci Rep. 2006. PMID: 16469265 Review.
Cited by
-
Remarkable cell recovery from cerebral ischemia in rats using an adaptive escalator-based rehabilitation mechanism.PLoS One. 2019 Oct 11;14(10):e0223820. doi: 10.1371/journal.pone.0223820. eCollection 2019. PLoS One. 2019. PMID: 31603928 Free PMC article.
-
The Ethanolic Extract of Caesalpinia sappan Heartwood Inhibits Cerebral Ischemia/Reperfusion Injury in a Rat Model Through a Multi-Targeted Pharmacological Mechanism.Front Pharmacol. 2019 Feb 5;10:29. doi: 10.3389/fphar.2019.00029. eCollection 2019. Front Pharmacol. 2019. PMID: 30804781 Free PMC article.
-
RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways.Cell Death Dis. 2017 Oct 5;8(10):e3080. doi: 10.1038/cddis.2017.465. Cell Death Dis. 2017. PMID: 28981095 Free PMC article.
-
Protective effect of polysaccharide peptide on cerebral ischemia‑reperfusion injury in rats.Mol Med Rep. 2018 Dec;18(6):5371-5378. doi: 10.3892/mmr.2018.9579. Epub 2018 Oct 23. Mol Med Rep. 2018. PMID: 30365125 Free PMC article.
-
Expression of miR-200c corresponds with increased reactive oxygen species and hypoxia markers after transient focal ischemia in mice.Neurochem Int. 2021 Oct;149:105146. doi: 10.1016/j.neuint.2021.105146. Epub 2021 Jul 31. Neurochem Int. 2021. PMID: 34343653 Free PMC article.
References
-
- Abulrob A, Brunette E, Slinn J, Baumann E, Stanimirovic D. Dynamic analysis of the blood-brain barrier disruption in experimental stroke using time domain in vivo fluorescence imaging. Mol Imaging. 2008;7:248–262. - PubMed
-
- Aggoun-Zouaoui D, Margalli I, Borrega F, Represa A, Plotkine M, Ben-Ari Y, Charriaut-Marlangue C. Ultrastructural morphology of neuronal death following reversible focal ischemia in the rat. Apoptosis. 1998;3:133–141. - PubMed
-
- Auer RN, Kalimo H, Olsson Y, Siesjo BK. The temporal evolution of hypoglycemic brain damage. I. Light- and electron-microscopic findings in the rat cerebral cortex. Acta Neuropathol. 1985;67:13–24. - PubMed
-
- Back T, Hemmen T, Schuler OG. Lesion evolution in cerebral ischemia. J Neurol. 2004;251:388–397. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

