Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl iron complexes
- PMID: 22084240
- PMCID: PMC3249115
- DOI: 10.1074/jbc.M111.310987
Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl iron complexes
Abstract
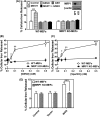
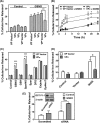
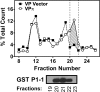
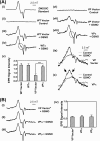
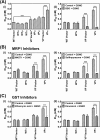
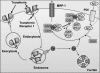
Nitrogen monoxide (NO) plays a role in the cytotoxic mechanisms of activated macrophages against tumor cells by inducing iron release. We showed that NO-mediated iron efflux from cells required glutathione (GSH) (Watts, R. N., and Richardson, D. R. (2001) J. Biol. Chem. 276, 4724-4732) and that the GSH-conjugate transporter, multidrug resistance-associated protein 1 (MRP1), mediates this release potentially as a dinitrosyl-dithiol iron complex (DNIC; Watts, R. N., Hawkins, C., Ponka, P., and Richardson, D. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7670-7675). Recently, glutathione S-transferase P1-1 (GST P1-1) was shown to bind DNICs as dinitrosyl-diglutathionyl iron complexes. Considering this and that GSTs and MRP1 form an integrated detoxification unit with chemotherapeutics, we assessed whether these proteins coordinately regulate storage and transport of DNICs as long lived NO intermediates. Cells transfected with GSTP1 (but not GSTA1 or GSTM1) significantly decreased NO-mediated 59Fe release from cells. This NO-mediated 59Fe efflux and the effect of GST P1-1 on preventing this were observed with NO-generating agents and also in cells transfected with inducible nitric oxide synthase. Notably, 59Fe accumulated in cells within GST P1-1-containing fractions, indicating an alteration in intracellular 59Fe distribution. Furthermore, electron paramagnetic resonance studies showed that MCF7-VP cells transfected with GSTP1 contain significantly greater levels of a unique DNIC signal. These investigations indicate that GST P1-1 acts to sequester NO as DNICs, reducing their transport out of the cell by MRP1. Cell proliferation studies demonstrated the importance of the combined effect of GST P1-1 and MRP1 in protecting cells from the cytotoxic effects of NO. Thus, the DNIC storage function of GST P1-1 and ability of MRP1 to efflux DNICs are vital in protection against NO cytotoxicity.
Figures

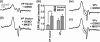
Similar articles
-
A Nitric Oxide Storage and Transport System That Protects Activated Macrophages from Endogenous Nitric Oxide Cytotoxicity.J Biol Chem. 2016 Dec 30;291(53):27042-27061. doi: 10.1074/jbc.M116.763714. Epub 2016 Nov 19. J Biol Chem. 2016. PMID: 27866158 Free PMC article.
-
Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1.Proc Natl Acad Sci U S A. 2006 May 16;103(20):7670-5. doi: 10.1073/pnas.0602515103. Epub 2006 May 5. Proc Natl Acad Sci U S A. 2006. PMID: 16679408 Free PMC article.
-
Nitrogen monoxide (NO) storage and transport by dinitrosyl-dithiol-iron complexes: long-lived NO that is trafficked by interacting proteins.J Biol Chem. 2012 Mar 2;287(10):6960-8. doi: 10.1074/jbc.R111.329847. Epub 2012 Jan 19. J Biol Chem. 2012. PMID: 22262835 Free PMC article.
-
Glutathione S-transferase and MRP1 form an integrated system involved in the storage and transport of dinitrosyl-dithiolato iron complexes in cells.Free Radic Biol Med. 2014 Oct;75:14-29. doi: 10.1016/j.freeradbiomed.2014.07.002. Epub 2014 Jul 15. Free Radic Biol Med. 2014. PMID: 25035074 Review.
-
The nitric oxide-iron interplay in mammalian cells: transport and storage of dinitrosyl iron complexes.Biochim Biophys Acta. 2008 Apr;1780(4):638-51. doi: 10.1016/j.bbagen.2007.12.009. Epub 2008 Jan 16. Biochim Biophys Acta. 2008. PMID: 18206118 Review.
Cited by
-
Detection of dinitrosyl iron complexes by ozone-based chemiluminescence.Nitric Oxide. 2018 Sep 1;79:57-67. doi: 10.1016/j.niox.2018.07.005. Epub 2018 Jul 27. Nitric Oxide. 2018. PMID: 30059767 Free PMC article.
-
A Nitric Oxide Storage and Transport System That Protects Activated Macrophages from Endogenous Nitric Oxide Cytotoxicity.J Biol Chem. 2016 Dec 30;291(53):27042-27061. doi: 10.1074/jbc.M116.763714. Epub 2016 Nov 19. J Biol Chem. 2016. PMID: 27866158 Free PMC article.
-
Glutathione-S-Transferases as Potential Targets for Modulation of Nitric Oxide-Mediated Vasodilation.Biomolecules. 2022 Sep 13;12(9):1292. doi: 10.3390/biom12091292. Biomolecules. 2022. PMID: 36139130 Free PMC article.
-
Iron deficiency triggered transcriptome changes in bread wheat.Comput Struct Biotechnol J. 2020 Sep 20;18:2709-2722. doi: 10.1016/j.csbj.2020.09.009. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33101609 Free PMC article.
-
The Chemical Biology of NO that Regulates Oncogenic Signaling and Metabolism: NOS2 and Its Role in Inflammatory Disease.Crit Rev Oncog. 2023;28(1):27-45. doi: 10.1615/CritRevOncog.2023047302. Crit Rev Oncog. 2023. PMID: 37824385 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

