Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP
- PMID: 22084027
- PMCID: PMC3378700
- DOI: 10.1002/stem.783
Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP
Abstract
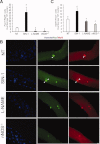
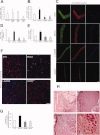
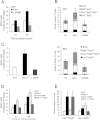
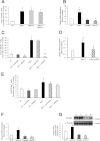
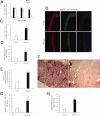
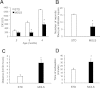
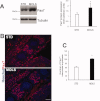
Satellite cells are myogenic precursors that proliferate, activate, and differentiate on muscle injury to sustain the regenerative capacity of adult skeletal muscle; in this process, they self-renew through the return to quiescence of the cycling progeny. This mechanism, while efficient in physiological conditions does not prevent exhaustion of satellite cells in pathologies such as muscular dystrophy where numerous rounds of damage occur. Here, we describe a key role of nitric oxide, an important signaling molecule in adult skeletal muscle, on satellite cells maintenance, studied ex vivo on isolated myofibers and in vivo using the α-sarcoglycan null mouse model of dystrophy and a cardiotoxin-induced model of repetitive damage. Nitric oxide stimulated satellite cells proliferation in a pathway dependent on cGMP generation. Furthermore, it increased the number of Pax7(+)/Myf5(-) cells in a cGMP-independent pathway requiring enhanced expression of Vangl2, a member of the planar cell polarity pathway involved in the Wnt noncanonical pathway. The enhanced self-renewal ability of satellite cells induced by nitric oxide is sufficient to delay the reduction of the satellite cell pool during repetitive acute and chronic damages, favoring muscle regeneration; in the α-sarcoglycan null dystrophic mouse, it also slowed disease progression persistently. These results identify nitric oxide as a key messenger in satellite cells maintenance, expand the significance of the Vangl2-dependent Wnt noncanonical pathway in myogenesis, and indicate novel strategies to optimize nitric oxide-based therapies for muscular dystrophy.
Copyright © 2011 AlphaMed Press.
Figures
Similar articles
-
Correlated NOS-Imu and myf5 expression by satellite cells in mdx mouse muscle regeneration during NOS manipulation and deflazacort treatment.Neuromuscul Disord. 2003 Jun;13(5):388-96. doi: 10.1016/s0960-8966(03)00029-4. Neuromuscul Disord. 2003. PMID: 12798794
-
Development of a nitric oxide-releasing analogue of the muscle relaxant guaifenesin for skeletal muscle satellite cell myogenesis.Mol Pharm. 2009 May-Jun;6(3):895-904. doi: 10.1021/mp800226z. Mol Pharm. 2009. PMID: 19317416
-
Canonical NF-κB signaling regulates satellite stem cell homeostasis and function during regenerative myogenesis.J Mol Cell Biol. 2019 Jan 1;11(1):53-66. doi: 10.1093/jmcb/mjy053. J Mol Cell Biol. 2019. PMID: 30239789
-
Myogenic Satellite Cells: Biological Milieu and Possible Clinical Applications.Pak J Biol Sci. 2017;20(1):1-11. doi: 10.3923/pjbs.2017.1.11. Pak J Biol Sci. 2017. PMID: 29023009 Review.
-
Niche regulation of muscle satellite cell self-renewal and differentiation.Cell Stem Cell. 2008 Jan 10;2(1):22-31. doi: 10.1016/j.stem.2007.12.012. Cell Stem Cell. 2008. PMID: 18371418 Review.
Cited by
-
Nitric Oxide Donor Molsidomine Positively Modulates Myogenic Differentiation of Embryonic Endothelial Progenitors.PLoS One. 2016 Oct 19;11(10):e0164893. doi: 10.1371/journal.pone.0164893. eCollection 2016. PLoS One. 2016. PMID: 27760216 Free PMC article.
-
Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage.J Immunol. 2013 Feb 15;190(4):1767-77. doi: 10.4049/jimmunol.1202903. Epub 2013 Jan 18. J Immunol. 2013. PMID: 23335752 Free PMC article.
-
Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models.Int J Mol Sci. 2022 Nov 2;23(21):13380. doi: 10.3390/ijms232113380. Int J Mol Sci. 2022. PMID: 36362166 Free PMC article. Review.
-
Mitochondrial Function and Reactive Oxygen/Nitrogen Species in Skeletal Muscle.Front Cell Dev Biol. 2022 Feb 21;10:826981. doi: 10.3389/fcell.2022.826981. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35265618 Free PMC article. Review.
-
Systemic Actions of Breast Cancer Facilitate Functional Limitations.Cancers (Basel). 2020 Jan 13;12(1):194. doi: 10.3390/cancers12010194. Cancers (Basel). 2020. PMID: 31941005 Free PMC article. Review.
References
-
- Paylor B, Natarajan A, Zhang RH, et al. Nonmyogenic cells in skeletal muscle regeneration. Curr Top Dev Biol. 2011;96:139–165. - PubMed
-
- Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. - PubMed
-
- Buckingham M, Bajard L, Daubas P, et al. Myogenic progenitor cells in the mouse embryo are marked by the expression of Pax3/7 genes that regulate their survival and myogenic potential. Anat Embryol. 2006;211(suppl 1):51–56. - PubMed
-
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. - PubMed
-
- Fukada S, Uezumi A, Ikemoto M, et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

