Novel protein substrates of the phospho-form modification system in Neisseria gonorrhoeae and their connection to O-linked protein glycosylation
- PMID: 22083701
- PMCID: PMC3255651
- DOI: 10.1128/IAI.05920-11
Novel protein substrates of the phospho-form modification system in Neisseria gonorrhoeae and their connection to O-linked protein glycosylation
Abstract
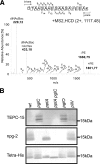
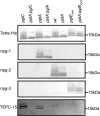
The zwitterionic phospho-form moieties phosphoethanolamine (PE) and phosphocholine (PC) are important components of bacterial membranes and cell surfaces. The major type IV pilus subunit protein of Neisseria gonorrhoeae, PilE, undergoes posttranslational modifications with these moieties via the activity of the pilin phospho-form transferase PptA. A number of observations relating to colocalization of phospho-form and O-linked glycan attachment sites in PilE suggested that these modifications might be either functionally or mechanistically linked or interact directly or indirectly. Moreover, it was unknown whether the phenomenon of phospho-form modification was solely dedicated to PilE or if other neisserial protein targets might exist. In light of these concerns, we screened for evidence of phospho-form modification on other membrane glycoproteins targeted by the broad-spectrum O-linked glycosylation system. In this way, two periplasmic lipoproteins, NGO1043 and NGO1237, were identified as substrates for PE addition. As seen previously for PilE, sites of PE modifications were clustered with those of glycan attachment. In the case of NGO1043, evidence for at least six serine phospho-form attachment sites was found, and further analyses revealed that at least two of these serines were also attachment sites for glycan. Finally, mutations altering glycosylation status led to the presence of pptA-dependent PC modifications on both proteins. Together, these results reinforce the associations established in PilE and provide evidence for dynamic interplay between phospho-form modification and O-linked glycosylation. The observations also suggest that phospho-form modifications likely contribute biologically at both intracellular and extracellular levels.
Figures


Similar articles
-
Type IV pilus assembly proficiency and dynamics influence pilin subunit phospho-form macro- and microheterogeneity in Neisseria gonorrhoeae.PLoS One. 2014 May 5;9(5):e96419. doi: 10.1371/journal.pone.0096419. eCollection 2014. PLoS One. 2014. PMID: 24797914 Free PMC article.
-
Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases.J Biol Chem. 2006 Sep 22;281(38):27712-23. doi: 10.1074/jbc.M604324200. Epub 2006 Jul 5. J Biol Chem. 2006. PMID: 16825186
-
Neisseria gonorrhoeae O-linked pilin glycosylation: functional analyses define both the biosynthetic pathway and glycan structure.Mol Microbiol. 2007 Aug;65(3):607-24. doi: 10.1111/j.1365-2958.2007.05806.x. Epub 2007 Jun 29. Mol Microbiol. 2007. PMID: 17608667 Free PMC article.
-
Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae--a review.Gene. 1997 Jun 11;192(1):125-34. doi: 10.1016/s0378-1119(97)00038-3. Gene. 1997. PMID: 9224882 Review.
-
Understanding protein glycosylation pathways in bacteria.Future Microbiol. 2017 Jan;12:59-72. doi: 10.2217/fmb-2016-0166. Epub 2016 Sep 30. Future Microbiol. 2017. PMID: 27689684 Review.
Cited by
-
Isolation and characterization of Neisseria musculi sp. nov., from the wild house mouse.Int J Syst Evol Microbiol. 2016 Sep;66(9):3585-3593. doi: 10.1099/ijsem.0.001237. Epub 2016 Jun 13. Int J Syst Evol Microbiol. 2016. PMID: 27298306 Free PMC article.
-
Sculpting the Bacterial O-Glycoproteome: Functional Analyses of Orthologous Oligosaccharyltransferases with Diverse Targeting Specificities.mBio. 2022 Jun 28;13(3):e0379721. doi: 10.1128/mbio.03797-21. Epub 2022 Apr 26. mBio. 2022. PMID: 35471082 Free PMC article.
-
Phosphoethanolamine Transferases as Drug Discovery Targets for Therapeutic Treatment of Multi-Drug Resistant Pathogenic Gram-Negative Bacteria.Antibiotics (Basel). 2023 Aug 29;12(9):1382. doi: 10.3390/antibiotics12091382. Antibiotics (Basel). 2023. PMID: 37760679 Free PMC article. Review.
-
Structural and Functional Characterization of the BcsG Subunit of the Cellulose Synthase in Salmonella typhimurium.J Mol Biol. 2018 Sep 14;430(18 Pt B):3170-3189. doi: 10.1016/j.jmb.2018.07.008. Epub 2018 Jul 12. J Mol Biol. 2018. PMID: 30017920 Free PMC article.
-
Mammalian Atg8 proteins and the autophagy factor IRGM control mTOR and TFEB at a regulatory node critical for responses to pathogens.Nat Cell Biol. 2020 Aug;22(8):973-985. doi: 10.1038/s41556-020-0549-1. Epub 2020 Aug 3. Nat Cell Biol. 2020. PMID: 32753672 Free PMC article.
References
-
- Aas FE, et al. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 281: 27712–27723 - PubMed
-
- Balatri E, Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. 2003. Solution structure of Sco1: a thioredoxin-like protein involved in cytochrome c oxidase assembly. Structure 11: 1431–1443 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

