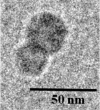
CRLX101 (formerly IT-101)-A Novel Nanopharmaceutical of Camptothecin in Clinical Development
- PMID: 22081768
- PMCID: PMC3182091
- DOI: 10.2174/157340711795163866
CRLX101 (formerly IT-101)-A Novel Nanopharmaceutical of Camptothecin in Clinical Development
Abstract
CRLX101 (formerly IT-101) is a first-in-class nanopharmaceutical, currently in Phase 2a development, which has been developed by covalently conjugating camptothecin (CPT) to a linear, cyclodextrin-polyethylene glycol (CD-PEG) co-polymer that self-assembles into nanoparticles. As a nanometer-scale drug carrier system, the cyclodextrin polymeric nanoparticle technology, referred to as "CDP", has unique design features and capabilities. Specifically, CRLX101 preclinical and clinical data confirm that CDP can address not only solubility, formulation, toxicity, and pharmacokinetic challenges associated with administration of CPT, but more importantly, can impart unique biological properties that enhance CPT pharmacodynamics and efficacy.
Figures
Similar articles
-
Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101.J Control Release. 2011 Jul 15;153(1):49-55. doi: 10.1016/j.jconrel.2011.03.007. Epub 2011 Mar 23. J Control Release. 2011. PMID: 21406204
-
Preclinical effects of CRLX101, an investigational camptothecin-containing nanoparticle drug conjugate, on treating glioblastoma multiforme via apoptosis and antiangiogenesis.Oncotarget. 2016 Jul 5;7(27):42408-42421. doi: 10.18632/oncotarget.9878. Oncotarget. 2016. PMID: 27285755 Free PMC article.
-
First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies.Invest New Drugs. 2013 Aug;31(4):986-1000. doi: 10.1007/s10637-012-9921-8. Epub 2013 Feb 9. Invest New Drugs. 2013. PMID: 23397498 Free PMC article. Clinical Trial.
-
A Systematic Review of Clinical Trials on the Efficacy and Safety of CRLX101 Cyclodextrin-Based Nanomedicine for Cancer Treatment.Pharmaceutics. 2023 Jun 26;15(7):1824. doi: 10.3390/pharmaceutics15071824. Pharmaceutics. 2023. PMID: 37514011 Free PMC article. Review.
-
Design and development of IT-101, a cyclodextrin-containing polymer conjugate of camptothecin.Adv Drug Deliv Rev. 2009 Nov 12;61(13):1189-92. doi: 10.1016/j.addr.2009.05.005. Epub 2009 Aug 12. Adv Drug Deliv Rev. 2009. PMID: 19682514 Review.
Cited by
-
Nanoparticles in Clinical Translation for Cancer Therapy.Int J Mol Sci. 2022 Feb 1;23(3):1685. doi: 10.3390/ijms23031685. Int J Mol Sci. 2022. PMID: 35163607 Free PMC article. Review.
-
Recent developments in topoisomerase-targeted cancer chemotherapy.Acta Pharm Sin B. 2018 Oct;8(6):844-861. doi: 10.1016/j.apsb.2018.07.008. Epub 2018 Jul 25. Acta Pharm Sin B. 2018. PMID: 30505655 Free PMC article. Review.
-
Chemotherapy Based on Supramolecular Chemistry: A Promising Strategy in Cancer Therapy.Pharmaceutics. 2019 Jun 20;11(6):292. doi: 10.3390/pharmaceutics11060292. Pharmaceutics. 2019. PMID: 31226856 Free PMC article. Review.
-
CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α.Cancer Res. 2017 Jan 1;77(1):112-122. doi: 10.1158/0008-5472.CAN-15-2951. Epub 2016 Oct 26. Cancer Res. 2017. PMID: 27784746 Free PMC article.
-
Multilayered polymer-coated carbon nanotubes to deliver dasatinib.Mol Pharm. 2014 Jan 6;11(1):276-82. doi: 10.1021/mp400448w. Epub 2013 Dec 9. Mol Pharm. 2014. PMID: 24294824 Free PMC article.
References
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. - PubMed
-
- Huang SK, Mayhew E, Gilani S, Lasic DD, Martin FJ, Papahadjopoulos D. Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon carcinoma. Cancer Res. 1992;52:6774–6781. - PubMed
-
- Tong R, Cheng J. Anticancer polymeric nanomedicines. Polymer Rev. 2007;47:345–381.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials