Improving delivery and efficacy of nanomedicines in solid tumors: role of tumor priming
- PMID: 22077464
- PMCID: PMC3655409
- DOI: 10.2217/nnm.11.141
Improving delivery and efficacy of nanomedicines in solid tumors: role of tumor priming
Abstract
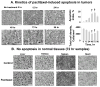
Effectiveness of nanomedicines in cancer therapy is limited in part by inadequate delivery and transport in tumor interstitium. This article reviews the experimental approaches to improve nanomedicine delivery and transport in solid tumors. These approaches include tumor vasculature normalization, interstitial fluid pressure modulation, enzymatic extracellular matrix degradation, and apoptosis-inducing tumor priming technology. We advocate the latter approach due to its ease and practicality (accomplished with standard-of-care chemotherapy, such as paclitaxel) and tumor selectivity. Examples of applying tumor priming to deliver nanomedicines and to design drug/RNAi-loaded carriers are discussed.
Figures




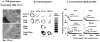
Similar articles
-
Tumor priming enhances delivery and efficacy of nanomedicines.J Pharmacol Exp Ther. 2007 Jul;322(1):80-8. doi: 10.1124/jpet.107.121632. Epub 2007 Apr 9. J Pharmacol Exp Ther. 2007. PMID: 17420296
-
Combining Nanomedicine and Immunotherapy.Acc Chem Res. 2019 Jun 18;52(6):1543-1554. doi: 10.1021/acs.accounts.9b00148. Epub 2019 May 23. Acc Chem Res. 2019. PMID: 31120725 Free PMC article.
-
Hyperthermia approaches for enhanced delivery of nanomedicines to solid tumors.Biotechnol Bioeng. 2015 Oct;112(10):1967-83. doi: 10.1002/bit.25653. Epub 2015 Jul 14. Biotechnol Bioeng. 2015. PMID: 25995079 Review.
-
Strategies to improve tumor penetration of nanomedicines through nanoparticle design.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019 Jan;11(1):e1519. doi: 10.1002/wnan.1519. Epub 2018 Apr 16. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019. PMID: 29659166 Review.
-
Recent Advances in Targeted Tumor Chemotherapy Based on Smart Nanomedicines.Small. 2018 Nov;14(45):e1802417. doi: 10.1002/smll.201802417. Epub 2018 Sep 3. Small. 2018. PMID: 30247806 Review.
Cited by
-
Complex effects of tumor microenvironment on the tumor disposition of carrier-mediated agents.Nanomedicine (Lond). 2017 Aug;12(16):2021-2042. doi: 10.2217/nnm-2017-0101. Epub 2017 Jul 26. Nanomedicine (Lond). 2017. PMID: 28745129 Free PMC article. Review.
-
Paclitaxel-loaded polymeric microparticles: quantitative relationships between in vitro drug release rate and in vivo pharmacodynamics.J Control Release. 2013 Dec 28;172(3):737-44. doi: 10.1016/j.jconrel.2013.09.011. Epub 2013 Sep 20. J Control Release. 2013. PMID: 24056144 Free PMC article.
-
Multiscale tumor spatiokinetic model for intraperitoneal therapy.AAPS J. 2014 May;16(3):424-39. doi: 10.1208/s12248-014-9574-y. Epub 2014 Feb 26. AAPS J. 2014. PMID: 24570339 Free PMC article.
-
Systemic Bioequivalence Is Unlikely to Equal Target Site Bioequivalence for Nanotechnology Oncologic Products.AAPS J. 2019 Feb 1;21(2):24. doi: 10.1208/s12248-019-0296-z. AAPS J. 2019. PMID: 30710324 Free PMC article. Review.
-
Target Site Delivery and Residence of Nanomedicines: Application of Quantitative Systems Pharmacology.Pharmacol Rev. 2019 Apr;71(2):157-169. doi: 10.1124/pr.118.016816. Pharmacol Rev. 2019. PMID: 30846487 Free PMC article. Review.
References
-
- Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363(25):2434–2443. - PubMed
-
- Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19(3):311–330. - PubMed
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. - PubMed
-
- Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. - PubMed
-
- Zhang JA, Xuan T, Parmar M, et al. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm. 2004;270(1–2):93–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R43CA121744/CA/NCI NIH HHS/United States
- R01CA123159/CA/NCI NIH HHS/United States
- R43CA134047/CA/NCI NIH HHS/United States
- R44CA103133/CA/NCI NIH HHS/United States
- R44 CA103133/CA/NCI NIH HHS/United States
- R43 CA121744/CA/NCI NIH HHS/United States
- R01EB015253/EB/NIBIB NIH HHS/United States
- R21 CA100553/CA/NCI NIH HHS/United States
- R43 CA134047/CA/NCI NIH HHS/United States
- R01 CA158300/CA/NCI NIH HHS/United States
- R01CA158300/CA/NCI NIH HHS/United States
- R01 EB015253/EB/NIBIB NIH HHS/United States
- R01 CA123159/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
