Evidence against extracellular exposure of a highly immunogenic region in the C-terminal domain of the simian immunodeficiency virus gp41 transmembrane protein
- PMID: 22072749
- PMCID: PMC3255797
- DOI: 10.1128/JVI.06463-11
Evidence against extracellular exposure of a highly immunogenic region in the C-terminal domain of the simian immunodeficiency virus gp41 transmembrane protein
Abstract
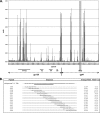
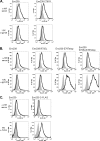
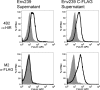
The generally accepted model for human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein topology includes a single membrane-spanning domain. An alternate model has been proposed which features multiple membrane-spanning domains. Consistent with the alternate model, a high percentage of HIV-1-infected individuals produce unusually robust antibody responses to a region of envelope, the so-called "Kennedy epitope," that in the conventional model should be in the cytoplasm. Here we show analogous, robust antibody responses in simian immunodeficiency virus SIVmac239-infected rhesus macaques to a region of SIVmac239 envelope located in the C-terminal domain, which in the conventional model should be inside the cell. Sera from SIV-infected rhesus macaques consistently reacted with overlapping oligopeptides corresponding to a region located within the cytoplasmic domain of gp41 by the generally accepted model, at intensities comparable to those observed for immunodominant areas of the surface component gp120. Rabbit serum raised against this highly immunogenic region (HIR) reacted with SIV envelope in cell surface-staining experiments, as did monoclonal anti-HIR antibodies isolated from an SIVmac239-infected rhesus macaque. However, control experiments demonstrated that this surface staining could be explained in whole or in part by the release of envelope protein from expressing cells into the supernatant and the subsequent attachment to the surfaces of cells in the culture. Serum and monoclonal antibodies directed against the HIR failed to neutralize even the highly neutralization-sensitive strain SIVmac316. Furthermore, a potential N-linked glycosylation site located close to the HIR and postulated to be outside the cell in the alternate model was not glycosylated. An artificially introduced glycosylation site within the HIR was also not utilized for glycosylation. Together, these data support the conventional model of SIV envelope as a type Ia transmembrane protein with a single membrane-spanning domain and without any extracellular loops.
Figures

Similar articles
-
The intracytoplasmic domain of the Env transmembrane protein is a locus for attenuation of simian immunodeficiency virus SIVmac in rhesus macaques.J Virol. 2000 Jul;74(13):5836-44. doi: 10.1128/jvi.74.13.5836-5844.2000. J Virol. 2000. PMID: 10846063 Free PMC article.
-
An epitope on the surface envelope glycoprotein (gp130) of simian immunodeficiency virus (SIVmac) involved in viral neutralization and T cell activation.AIDS Res Hum Retroviruses. 1993 May;9(5):423-30. doi: 10.1089/aid.1993.9.423. AIDS Res Hum Retroviruses. 1993. PMID: 7686386
-
Importance of the intracytoplasmic domain of the simian immunodeficiency virus (SIV) envelope glycoprotein for pathogenesis.Virology. 1998 Dec 5;252(1):9-16. doi: 10.1006/viro.1998.9467. Virology. 1998. PMID: 9875311
-
Neutralising epitopes of simian immunodeficiency virus envelope glycoprotein.J Med Primatol. 1995 May;24(3):145-9. doi: 10.1111/j.1600-0684.1995.tb00160.x. J Med Primatol. 1995. PMID: 8751054 Review.
-
Antigenic and immunogenic sites of HIV-2 glycoproteins.Chem Immunol. 1993;56:61-77. doi: 10.1159/000319156. Chem Immunol. 1993. PMID: 8452654 Review.
Cited by
-
Distinct functions for the membrane-proximal ectodomain region (MPER) of HIV-1 gp41 in cell-free and cell-cell viral transmission and cell-cell fusion.J Biol Chem. 2018 Apr 20;293(16):6099-6120. doi: 10.1074/jbc.RA117.000537. Epub 2018 Mar 1. J Biol Chem. 2018. PMID: 29496992 Free PMC article.
-
The tale of the long tail: the cytoplasmic domain of HIV-1 gp41.J Virol. 2013 Jan;87(1):2-15. doi: 10.1128/JVI.02053-12. Epub 2012 Oct 17. J Virol. 2013. PMID: 23077317 Free PMC article. Review.
-
The cytoplasmic tail of retroviral envelope glycoproteins.Prog Mol Biol Transl Sci. 2015;129:253-84. doi: 10.1016/bs.pmbts.2014.10.009. Epub 2014 Dec 1. Prog Mol Biol Transl Sci. 2015. PMID: 25595807 Free PMC article. Review.
-
Structural and functional comparisons of retroviral envelope protein C-terminal domains: still much to learn.Viruses. 2014 Jan 16;6(1):284-300. doi: 10.3390/v6010284. Viruses. 2014. PMID: 24441863 Free PMC article. Review.
-
The frantic play of the concealed HIV envelope cytoplasmic tail.Retrovirology. 2013 May 24;10:54. doi: 10.1186/1742-4690-10-54. Retrovirology. 2013. PMID: 23705972 Free PMC article. Review.
References
-
- Boge M, Wyss S, Bonifacino JS, Thali M. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773–15778 - PubMed
-
- Bowers K, et al. 2000. The simian immunodeficiency virus envelope glycoprotein contains multiple signals that regulate its cell surface expression and endocytosis. Traffic 1:661–674 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

