The effects of cholera toxin on cellular energy metabolism
- PMID: 22069603
- PMCID: PMC3153216
- DOI: 10.3390/toxins2040632
The effects of cholera toxin on cellular energy metabolism
Abstract
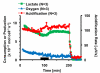
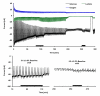
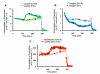
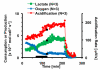
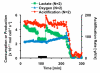
Multianalyte microphysiometry, a real-time instrument for simultaneous measurement of metabolic analytes in a microfluidic environment, was used to explore the effects of cholera toxin (CTx). Upon exposure of CTx to PC-12 cells, anaerobic respiration was triggered, measured as increases in acid and lactate production and a decrease in the oxygen uptake. We believe the responses observed are due to a CTx-induced activation of adenylate cyclase, increasing cAMP production and resulting in a switch to anaerobic respiration. Inhibitors (H-89, brefeldin A) and stimulators (forskolin) of cAMP were employed to modulate the CTx-induced cAMP responses. The results of this study show the utility of multianalyte microphysiometry to quantitatively determine the dynamic metabolic effects of toxins and affected pathways.
Keywords: PC-12; cholera toxin; cyclic AMP; forskolin; metabolism; microphysiometry; multianalyte.
Figures

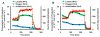
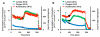

Similar articles
-
Multianalyte microphysiometry reveals changes in cellular bioenergetics upon exposure to fluorescent dyes.Anal Chem. 2013 Dec 17;85(24):11677-80. doi: 10.1021/ac402764x. Epub 2013 Nov 26. Anal Chem. 2013. PMID: 24228839 Free PMC article.
-
Guanine nucleotide binding proteins and the regulation of cyclic AMP synthesis in NS20Y neuroblastoma cells: role of D1 dopamine and muscarinic receptors.Brain Res. 1991 Aug 9;556(1):101-7. doi: 10.1016/0006-8993(91)90552-7. Brain Res. 1991. PMID: 1682005
-
Forskolin potentiation of cholera toxin-stimulated cyclic AMP accumulation in intact C6-2B cells. Evidence for enhanced Gs-C coupling.Mol Pharmacol. 1985 Dec;28(6):502-7. Mol Pharmacol. 1985. PMID: 3001496
-
Importance of ADP-ribosylation in the morphological changes of PC12 cells induced by cholera toxin.Infect Immun. 1994 Oct;62(10):4176-85. doi: 10.1128/iai.62.10.4176-4185.1994. Infect Immun. 1994. PMID: 7927673 Free PMC article.
-
Effects of follicle stimulating hormone, cholera toxin, pertussis toxin and forskolin on adenosine cyclic 3',5'-monophosphate output by granulosa cells from Booroola ewes with or without the F gene.J Reprod Fertil. 1990 Jul;89(2):553-63. doi: 10.1530/jrf.0.0890553. J Reprod Fertil. 1990. PMID: 2169531
Cited by
-
Engineering challenges for instrumenting and controlling integrated organ-on-chip systems.IEEE Trans Biomed Eng. 2013 Mar;60(3):682-90. doi: 10.1109/TBME.2013.2244891. Epub 2013 Feb 1. IEEE Trans Biomed Eng. 2013. PMID: 23380852 Free PMC article.
-
Mechanistic analysis of challenge-response experiments.Biometrics. 2013 Sep;69(3):741-7. doi: 10.1111/biom.12066. Epub 2013 Jul 16. Biometrics. 2013. PMID: 23859366 Free PMC article.
-
Multianalyte Microphysiometry of Macrophage Responses to Phorbol Myristate Acetate, Lipopolysaccharide, and Lipoarabinomannan.Electroanalysis. 2013 Jul 1;25(7):1706-1712. doi: 10.1002/elan.201300121. Electroanalysis. 2013. PMID: 25798034 Free PMC article.
-
Multichamber Multipotentiostat System for Cellular Microphysiometry.Sens Actuators B Chem. 2014 Dec 1;204:536-543. doi: 10.1016/j.snb.2014.07.126. Sens Actuators B Chem. 2014. PMID: 25242863 Free PMC article.
-
Multianalyte microphysiometry reveals changes in cellular bioenergetics upon exposure to fluorescent dyes.Anal Chem. 2013 Dec 17;85(24):11677-80. doi: 10.1021/ac402764x. Epub 2013 Nov 26. Anal Chem. 2013. PMID: 24228839 Free PMC article.
References
-
- Sack D.A., Sack R.B., Nair G.B., Siddique A.K. Cholera. Lancet. 2004;363:223–233. - PubMed
-
- Finkelstein R.A. In: Medical Microbiology. 4th. Baron S., editor. University of Texas Medical Branch; Galveston, TX, USA: 1996. - PubMed
-
- Lundgren O. Enteric nerves and diarrhoea. Pharmacol. Toxicol. 2002;90:109–120. - PubMed
-
- Lencer W.I., Tsai B. The intracellular voyage of cholera toxin: going retro. Trends Biochem. Sci. 2003;28:639–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

