Enhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cells
- PMID: 22065926
- PMCID: PMC3209422
Enhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cells
Retraction in
-
Withdrawal: Enhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cells.Mol Vis. 2013 Jun 6;19:1258. Print 2013. Mol Vis. 2013. PMID: 23761728 Free PMC article. No abstract available.
Abstract
Background: To specifically deliver paclitaxel (PTX) to retinoblastoma (RB) cells, the anionic surface-charged poly(lactic-co-glycolic acid) (PLGA) NPs loaded with paclitaxel were conjugated with epithelial cell adhesion molecule (EpCAM) antibody for enhancing site-specific intracellular delivery of paclitaxel against EpCAM overexpressing RB cells.
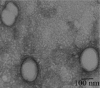
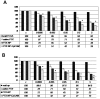
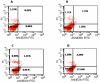
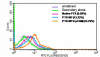
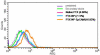
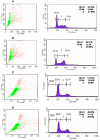
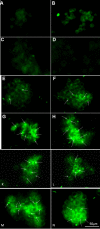
Methods: PTX-loaded PLGA NPs were prepared by the oil-in-water single emulsion solvent evaporation method, and the PTX content in NPs was estimated by the reverse phase isocratic mode of high performance liquid chromatography. Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride/N-hydroxysuccinimide chemistry was employed for the covalent attachment of monoclonal EpCAM antibody onto the NP surface. In vitro cytotoxicity of native PTX, unconjugated PTX-loaded NPs (PTX-NPs), and EpCAM antibody-conjugated PTX-loaded nanoparticles (PTX-NP-EpCAM) were evaluated on a Y79 RB cell line by a dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, while cellular apoptosis, cysteinyl-aspartic acid protease (caspase)-3 activation, Poly (adenosine diphosphate-ribose) polymerase (PARP) cleavage, and cell-cycle arrest were quantified by flow cytometry. By employing flow cytometry and fluorescence image analyses, the extent of cellular uptake was comparatively evaluated.
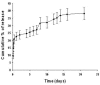
Results: PTX-NP-EpCAM had superior antiproliferation activity, increased arrested cell population at the G(2)-M phase, and increased activation of caspase-3, followed by PARP cleavage in parallel with the induction of apoptosis. Increased uptake of PTX-Np-EpCAM by the cells suggests that they were mainly taken up through EpCAM mediated endocytosis.
Conclusions: EpCAM antibody-functionalized biodegradable NPs for tumor-selective drug delivery and overcoming drug resistance could be an efficient therapeutic strategy for retinoblastoma treatment.
Figures




Similar articles
-
Novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold nanoparticles for enhanced and targeted small interfering RNA delivery to retinoblastoma cells.Mol Vis. 2013 May 6;19:1029-38. Print 2013. Mol Vis. 2013. PMID: 23687439 Free PMC article.
-
Synthesis, characterization, and evaluation of paclitaxel loaded in six-arm star-shaped poly(lactic-co-glycolic acid).Int J Nanomedicine. 2013;8:4315-26. doi: 10.2147/IJN.S51629. Epub 2013 Nov 7. Int J Nanomedicine. 2013. PMID: 24235829 Free PMC article.
-
Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy.Int J Nanomedicine. 2018 Sep 7;13:5231-5248. doi: 10.2147/IJN.S167142. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30237710 Free PMC article.
-
Enabling anticancer therapeutics by nanoparticle carriers: the delivery of Paclitaxel.Int J Mol Sci. 2011;12(7):4395-413. doi: 10.3390/ijms12074395. Epub 2011 Jul 7. Int J Mol Sci. 2011. PMID: 21845085 Free PMC article. Review.
-
Nanoparticle-mediated gene therapy as a novel strategy for the treatment of retinoblastoma.Colloids Surf B Biointerfaces. 2022 Dec;220:112899. doi: 10.1016/j.colsurfb.2022.112899. Epub 2022 Oct 4. Colloids Surf B Biointerfaces. 2022. PMID: 36252537 Review.
Cited by
-
Withdrawal: Enhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cells.Mol Vis. 2013 Jun 6;19:1258. Print 2013. Mol Vis. 2013. PMID: 23761728 Free PMC article. No abstract available.
-
Nanomedicines for back of the eye drug delivery, gene delivery, and imaging.Prog Retin Eye Res. 2013 Sep;36:172-98. doi: 10.1016/j.preteyeres.2013.04.001. Epub 2013 Apr 17. Prog Retin Eye Res. 2013. PMID: 23603534 Free PMC article. Review.
-
Critical evaluation of biodegradable polymers used in nanodrugs.Int J Nanomedicine. 2013;8:3071-90. doi: 10.2147/IJN.S47186. Epub 2013 Aug 19. Int J Nanomedicine. 2013. PMID: 23990720 Free PMC article. Review.
-
Novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold nanoparticles for enhanced and targeted small interfering RNA delivery to retinoblastoma cells.Mol Vis. 2013 May 6;19:1029-38. Print 2013. Mol Vis. 2013. PMID: 23687439 Free PMC article.
-
Molecular deregulation induced by silencing of the high mobility group protein A2 gene in retinoblastoma cells.Mol Vis. 2012;18:2420-37. Epub 2012 Oct 3. Mol Vis. 2012. PMID: 23077401 Free PMC article.
References
-
- Jain KK. Targeted drug delivery for cancer. Technol Cancer Res Treat. 2005;4:311–3. - PubMed
-
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–51. - PubMed
-
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. - PubMed
-
- Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8:1112–20. - PubMed
-
- Wang C, Ho PC, Lim LY. Wheat germ agglutinin-conjugated PLGA nanoparticles for enhanced intracellular delivery of paclitaxel to colon cancer cells. Int J Pharm. 2010;400:201–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
