TIN2 stability is regulated by the E3 ligase Siah2
- PMID: 22064479
- PMCID: PMC3255770
- DOI: 10.1128/MCB.06227-11
TIN2 stability is regulated by the E3 ligase Siah2
Abstract
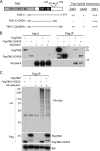
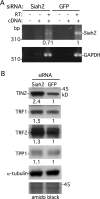
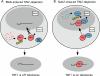
Telomeres are coated by shelterin, a six-subunit complex that is required for protection and replication of chromosome ends. The central subunit TIN2, with binding sites to three subunits (TRF1, TRF2, and TPP1), is essential for stability and function of the complex. Here we show that TIN2 stability is regulated by the E3 ligase Siah2. We demonstrate that TIN2 binds to Siah2 and is ubiquitylated in vivo. We show using purified proteins that Siah2 acts as an E3 ligase to directly ubiquitylate TIN2 in vitro. Depletion of Siah2 led to stabilization of TIN2 protein, indicating that Siah2 regulates TIN2 protein levels in vivo. Overexpression of Siah2 in human cells led to loss of TIN2 at telomeres that was dependent on the presence of the catalytic RING domain of Siah2. In contrast to RNAi-mediated depletion of TIN2 that led to loss of TRF1 and TRF2 at telomeres, Siah2-mediated depletion of TIN2 allowed TRF1 and TRF2 to remain on telomeres, indicating a different fate for shelterin subunits when TIN2 is depleted posttranslationally. TPP1 was lost from telomeres, although its protein level was not reduced. We speculate that Siah2-mediated removal of TIN2 may allow dynamic remodeling of the shelterin complex and its associated factors during the cell cycle.
Figures




Similar articles
-
TRF2-tethered TIN2 can mediate telomere protection by TPP1/POT1.Mol Cell Biol. 2014 Apr;34(7):1349-62. doi: 10.1128/MCB.01052-13. Epub 2014 Jan 27. Mol Cell Biol. 2014. PMID: 24469404 Free PMC article.
-
Human Telomere Repeat Binding Factor TRF1 Replaces TRF2 Bound to Shelterin Core Hub TIN2 when TPP1 Is Absent.J Mol Biol. 2019 Aug 9;431(17):3289-3301. doi: 10.1016/j.jmb.2019.05.038. Epub 2019 May 31. J Mol Biol. 2019. PMID: 31158366
-
Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex.Cell Res. 2017 Dec;27(12):1485-1502. doi: 10.1038/cr.2017.144. Epub 2017 Nov 21. Cell Res. 2017. PMID: 29160297 Free PMC article.
-
Shelterin: the protein complex that shapes and safeguards human telomeres.Genes Dev. 2005 Sep 15;19(18):2100-10. doi: 10.1101/gad.1346005. Genes Dev. 2005. PMID: 16166375 Review.
-
[Advance in research on the function of telomeric shelterin component TPP1 and its relationship with characteristics of tumors].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016 Aug;33(4):573-7. doi: 10.3760/cma.j.issn.1003-9406.2016.04.033. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016. PMID: 27455024 Review. Chinese.
Cited by
-
Seven in Absentia E3 Ubiquitin Ligases: Central Regulators of Neural Cell Fate and Neuronal Polarity.Front Cell Neurosci. 2017 Oct 13;11:322. doi: 10.3389/fncel.2017.00322. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29081737 Free PMC article. Review.
-
Ubiquitination and SUMOylation in Telomere Maintenance and Dysfunction.Front Genet. 2017 May 23;8:67. doi: 10.3389/fgene.2017.00067. eCollection 2017. Front Genet. 2017. PMID: 28588610 Free PMC article. Review.
-
Fusing telomeres with RNF8.Nucleus. 2012 Mar 1;3(2):143-9. doi: 10.4161/nucl.19322. Epub 2012 Mar 1. Nucleus. 2012. PMID: 22555600 Free PMC article. Review.
-
Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization.Nat Rev Mol Cell Biol. 2021 Apr;22(4):283-298. doi: 10.1038/s41580-021-00328-y. Epub 2021 Feb 9. Nat Rev Mol Cell Biol. 2021. PMID: 33564154 Free PMC article. Review.
-
Molecular basis of telomere dysfunction in human genetic diseases.Nat Struct Mol Biol. 2015 Nov;22(11):867-74. doi: 10.1038/nsmb.3093. Nat Struct Mol Biol. 2015. PMID: 26581521 Review.
References
-
- Bartel PL, Chien C, Sternglanz R, Fields S. 1993. Using the two-hybrid system to detect protein-protein interaction, p 153–179 In Harley D. A. (ed), Cellular interactions in development: a practical approach. IRL Press, Oxford, United Kingdom
-
- Baumann P, Cech TR. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
