TAp63γ enhances nucleotide excision repair through transcriptional regulation of DNA repair genes
- PMID: 22056305
- PMCID: PMC3348579
- DOI: 10.1016/j.dnarep.2011.10.016
TAp63γ enhances nucleotide excision repair through transcriptional regulation of DNA repair genes
Abstract
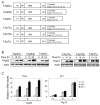
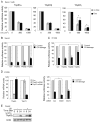
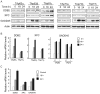
p63 and p73, two p53 family members, play crucial roles in development and tumor suppression. p63 and p73 have multiple isoforms, which have similar or distinct biological functions. Transactivation (TA) isoforms of p63 and p73 have high similarity with p53 and often have biological functions similar to p53. p53 plays an important role in nucleotide excision repair (NER) through transcriptional regulation of target genes involved in NER, including DDB2, XPC and GADD45. To investigate whether TAp63 and TAp73 play a similar role in NER, Saos2 cells with inducible expression of specific isoforms of TAp63 and TAp73, including TAp63α/β/γ and TAp73α/β/γ isoforms, were employed. Overexpression of TAp63γ significantly enhances NER of ultraviolet (UV)-induced DNA damage, including cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts, and enhances cell survival after UV irradiation in Soas2 cells. The enhancement of NER of UV-induced DNA damage by TAp63γ was also confirmed in H1299 cells with overexpression of TAp63γ. Consistently, knockdown of endogenous TAp63 decreases NER of UV-induced DNA damage in H1299 cells. TAp63α/β and TAp73α/β/γ isoforms do not have a clear effect on NER in Saos2 or H1299 cells. TAp63γ overexpression clearly induces the expression of DDB2, XPC and GADD45 at both RNA and protein levels. Furthermore, luciferase reporter assays show that TAp63γ transcriptionally activates DDB2, XPC and GADD45 genes through the regulation of the p53 binding elements in these genes. These results demonstrate that TAp63γ enhances NER to remove UV-induced DNA damage and maintain genomic stability through transcriptional induction of a set of NER proteins, which provides an additional important mechanism that contributes to the function of TAp63 in tumor suppression.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
p53 responsive nucleotide excision repair gene products p48 and XPC, but not p53, localize to sites of UV-irradiation-induced DNA damage, in vivo.Carcinogenesis. 2003 May;24(5):843-50. doi: 10.1093/carcin/bgg031. Carcinogenesis. 2003. PMID: 12771027
-
The DDB2 nucleotide excision repair gene product p48 enhances global genomic repair in p53 deficient human fibroblasts.DNA Repair (Amst). 2003 Jul 16;2(7):819-26. doi: 10.1016/s1568-7864(03)00066-1. DNA Repair (Amst). 2003. PMID: 12826282
-
The oncogenic phosphatase WIP1 negatively regulates nucleotide excision repair.DNA Repair (Amst). 2010 Jul 1;9(7):813-23. doi: 10.1016/j.dnarep.2010.04.005. Epub 2010 May 6. DNA Repair (Amst). 2010. PMID: 20451471 Free PMC article.
-
Structural diversity of p63 and p73 isoforms.Cell Death Differ. 2022 May;29(5):921-937. doi: 10.1038/s41418-022-00975-4. Epub 2022 Mar 21. Cell Death Differ. 2022. PMID: 35314772 Free PMC article. Review.
-
p53 and regulation of DNA damage recognition during nucleotide excision repair.DNA Repair (Amst). 2003 Sep 18;2(9):947-54. doi: 10.1016/s1568-7864(03)00087-9. DNA Repair (Amst). 2003. PMID: 12967652 Review.
Cited by
-
TAp63-Regulated miRNAs Suppress Cutaneous Squamous Cell Carcinoma through Inhibition of a Network of Cell-Cycle Genes.Cancer Res. 2020 Jun 15;80(12):2484-2497. doi: 10.1158/0008-5472.CAN-19-1892. Epub 2020 Mar 10. Cancer Res. 2020. PMID: 32156775 Free PMC article.
-
A protein with broad functions: damage-specific DNA-binding protein 2.Mol Biol Rep. 2022 Dec;49(12):12181-12192. doi: 10.1007/s11033-022-07963-4. Epub 2022 Oct 3. Mol Biol Rep. 2022. PMID: 36190612 Free PMC article. Review.
-
Signaling Pathways, Chemical and Biological Modulators of Nucleotide Excision Repair: The Faithful Shield against UV Genotoxicity.Oxid Med Cell Longev. 2019 Aug 7;2019:4654206. doi: 10.1155/2019/4654206. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31485292 Free PMC article. Review.
-
Transcriptional and Posttranslational Regulation of Nucleotide Excision Repair: The Guardian of the Genome against Ultraviolet Radiation.Int J Mol Sci. 2016 Nov 4;17(11):1840. doi: 10.3390/ijms17111840. Int J Mol Sci. 2016. PMID: 27827925 Free PMC article. Review.
-
A targeted gene expression platform allows for rapid analysis of chemical-induced antioxidant mRNA expression in zebrafish larvae.PLoS One. 2017 Feb 17;12(2):e0171025. doi: 10.1371/journal.pone.0171025. eCollection 2017. PLoS One. 2017. PMID: 28212397 Free PMC article.
References
-
- Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. - PubMed
-
- De Laurenzi VD, Catani MV, Terrinoni A, Corazzari M, Melino G, Costanzo A, Levrero M, Knight RA. Additional complexity in p73: induction by mitogens in lymphoid cells and identification of two new splicing variants epsilon and zeta. Cell Death Differ. 1999;6:389–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

