Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias
- PMID: 22045636
- PMCID: PMC3240715
- DOI: 10.1002/humu.21636
Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias
Abstract
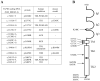
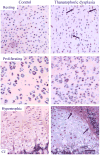
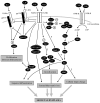
In 1994, the field of bone biology was significantly advanced by the discovery that activating mutations in the fibroblast growth factor receptor 3 (FGFR3) receptor tyrosine kinase (TK) account for the common genetic form of dwarfism in humans, achondroplasia (ACH). Other conditions soon followed, with the list of human disorders caused by FGFR3 mutations now reaching at least 10. An array of vastly different diagnoses is caused by similar mutations in FGFR3, including syndromes affecting skeletal development (hypochondroplasia [HCH], ACH, thanatophoric dysplasia [TD]), skin (epidermal nevi, seborrhaeic keratosis, acanthosis nigricans), and cancer (multiple myeloma [MM], prostate and bladder carcinoma, seminoma). Despite many years of research, several aspects of FGFR3 function in disease remain obscure or controversial. As FGFR3-related skeletal dysplasias are caused by growth attenuation of the cartilage, chondrocytes appear to be unique in their response to FGFR3 activation. However, the reasons why FGFR3 inhibits chondrocyte growth while causing excessive cellular proliferation in cancer are not clear. Likewise, the full spectrum of molecular events by which FGFR3 mediates its signaling is just beginning to emerge. This article describes the challenging journey to unravel the mechanisms of FGFR3 function in skeletal dysplasias, the extraordinary cellular manifestations of FGFR3 signaling in chondrocytes, and finally, the progress toward therapy for ACH and cancer.
© 2011 Wiley Periodicals, Inc.
Figures
Similar articles
-
Sustained phosphorylation of mutated FGFR3 is a crucial feature of genetic dwarfism and induces apoptosis in the ATDC5 chondrogenic cell line via PLCgamma-activated STAT1.Bone. 2007 Aug;41(2):273-81. doi: 10.1016/j.bone.2006.11.030. Epub 2007 Feb 9. Bone. 2007. PMID: 17561467
-
Meclozine facilitates proliferation and differentiation of chondrocytes by attenuating abnormally activated FGFR3 signaling in achondroplasia.PLoS One. 2013 Dec 4;8(12):e81569. doi: 10.1371/journal.pone.0081569. eCollection 2013. PLoS One. 2013. PMID: 24324705 Free PMC article.
-
Achondroplasia: Development, pathogenesis, and therapy.Dev Dyn. 2017 Apr;246(4):291-309. doi: 10.1002/dvdy.24479. Epub 2017 Mar 2. Dev Dyn. 2017. PMID: 27987249 Free PMC article. Review.
-
Hypochondroplasia, Acanthosis Nigricans, and Insulin Resistance in a Child with FGFR3 Mutation: Is It Just an Association?Case Rep Endocrinol. 2014;2014:840492. doi: 10.1155/2014/840492. Epub 2014 Nov 19. Case Rep Endocrinol. 2014. PMID: 25505998 Free PMC article.
-
The paradox of FGFR3 signaling in skeletal dysplasia: why chondrocytes growth arrest while other cells over proliferate.Mutat Res Rev Mutat Res. 2014 Jan-Mar;759:40-8. doi: 10.1016/j.mrrev.2013.11.001. Epub 2013 Dec 1. Mutat Res Rev Mutat Res. 2014. PMID: 24295726 Review.
Cited by
-
Deciphering the mutational signature of congenital limb malformations.Mol Ther Nucleic Acids. 2021 Apr 20;24:961-970. doi: 10.1016/j.omtn.2021.04.012. eCollection 2021 Jun 4. Mol Ther Nucleic Acids. 2021. PMID: 34094714 Free PMC article.
-
Fgfr3 mutation disrupts chondrogenesis and bone ossification in zebrafish model mimicking CATSHL syndrome partially via enhanced Wnt/β-catenin signaling.Theranostics. 2020 May 30;10(16):7111-7130. doi: 10.7150/thno.45286. eCollection 2020. Theranostics. 2020. PMID: 32641982 Free PMC article.
-
Pathogenic Cysteine Removal Mutations in FGFR Extracellular Domains Stabilize Receptor Dimers and Perturb the TM Dimer Structure.J Mol Biol. 2016 Oct 9;428(20):3903-3910. doi: 10.1016/j.jmb.2016.08.026. Epub 2016 Sep 3. J Mol Biol. 2016. PMID: 27596331 Free PMC article.
-
FGFR3 mutation frequency in 324 cases from the International Skeletal Dysplasia Registry.Mol Genet Genomic Med. 2014 Nov;2(6):497-503. doi: 10.1002/mgg3.96. Epub 2014 Aug 5. Mol Genet Genomic Med. 2014. PMID: 25614871 Free PMC article.
-
Clinical management and emerging therapies of FGFR3-related skeletal dysplasia in childhood.Ann Pediatr Endocrinol Metab. 2022 Jun;27(2):90-97. doi: 10.6065/apem.2244114.057. Epub 2022 Jun 30. Ann Pediatr Endocrinol Metab. 2022. PMID: 35793999 Free PMC article.
References
-
- Aikawa T, Segre GV, Lee K. Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E-Cdk2. J Biol Chem. 2001;276:29347–52. - PubMed
-
- Aviezer D, Golembo M, Yayon A. Fibroblast growth factor receptor-3 as a therapeutic target for achondroplasia-genetic short limbed dwarfism. Curr Drug Targets. 2003;4:353–65. - PubMed
-
- Avivi A, Zimmer Y, Yayon A, Yarden Y, Givol D. Flg-2, a new member of the family of fibroblast growth factor receptors. Oncogene. 1991;6:1089–92. - PubMed
-
- Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al-Gazali LI, et al. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. - PMC - PubMed
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

