Enterolobium contortisiliquum trypsin inhibitor (EcTI), a plant proteinase inhibitor, decreases in vitro cell adhesion and invasion by inhibition of Src protein-focal adhesion kinase (FAK) signaling pathways
- PMID: 22039045
- PMCID: PMC3249068
- DOI: 10.1074/jbc.M111.263996
Enterolobium contortisiliquum trypsin inhibitor (EcTI), a plant proteinase inhibitor, decreases in vitro cell adhesion and invasion by inhibition of Src protein-focal adhesion kinase (FAK) signaling pathways
Abstract
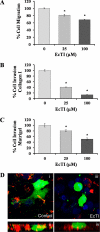
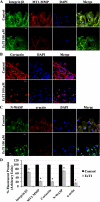
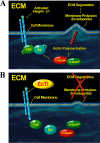
Tumor cell invasion is vital for cancer progression and metastasis. Adhesion, migration, and degradation of the extracellular matrix are important events involved in the establishment of cancer cells at a new site, and therefore molecular targets are sought to inhibit such processes. The effect of a plant proteinase inhibitor, Enterolobium contortisiliquum trypsin inhibitor (EcTI), on the adhesion, migration, and invasion of gastric cancer cells was the focus of this study. EcTI showed no effect on the proliferation of gastric cancer cells or fibroblasts but inhibited the adhesion, migration, and cell invasion of gastric cancer cells; however, EcTI had no effect upon the adhesion of fibroblasts. EcTI was shown to decrease the expression and disrupt the cellular organization of molecules involved in the formation and maturation of invadopodia, such as integrin β1, cortactin, neuronal Wiskott-Aldrich syndrome protein, membrane type 1 metalloprotease, and metalloproteinase-2. Moreover, gastric cancer cells treated with EcTI presented a significant decrease in intracellular phosphorylated Src and focal adhesion kinase, integrin-dependent cell signaling components. Together, these results indicate that EcTI inhibits the invasion of gastric cancer cells through alterations in integrin-dependent cell signaling pathways.
Figures


Similar articles
-
EcTI impairs survival and proliferation pathways in triple-negative breast cancer by modulating cell-glycosaminoglycans and inflammatory cytokines.Cancer Lett. 2020 Oct 28;491:108-120. doi: 10.1016/j.canlet.2020.08.017. Epub 2020 Aug 22. Cancer Lett. 2020. PMID: 32841713
-
The effects of a plant proteinase inhibitor from Enterolobium contortisiliquum on human tumor cell lines.Biol Chem. 2011 Apr;392(4):327-36. doi: 10.1515/BC.2011.031. Biol Chem. 2011. PMID: 21781023
-
Laminin-332-beta1 integrin interactions negatively regulate invadopodia.J Cell Physiol. 2010 Apr;223(1):134-42. doi: 10.1002/jcp.22018. J Cell Physiol. 2010. PMID: 20039268 Free PMC article.
-
The role of FAK in tumor metabolism and therapy.Pharmacol Ther. 2014 May;142(2):154-63. doi: 10.1016/j.pharmthera.2013.12.003. Epub 2013 Dec 9. Pharmacol Ther. 2014. PMID: 24333503 Free PMC article. Review.
-
Disrupting the scaffold to improve focal adhesion kinase-targeted cancer therapeutics.Sci Signal. 2013 Mar 26;6(268):pe10. doi: 10.1126/scisignal.2004021. Sci Signal. 2013. PMID: 23532331 Free PMC article. Review.
Cited by
-
Cutaneous Melanoma: An Overview of Physiological and Therapeutic Aspects and Biotechnological Use of Serine Protease Inhibitors.Molecules. 2024 Aug 16;29(16):3891. doi: 10.3390/molecules29163891. Molecules. 2024. PMID: 39202970 Free PMC article. Review.
-
3D Stroma Invasion Assay.Bio Protoc. 2017 Mar 20;7(6):e2195. doi: 10.21769/BioProtoc.2195. eCollection 2017 Mar 20. Bio Protoc. 2017. PMID: 34458502 Free PMC article.
-
Crystal structures of a plant trypsin inhibitor from Enterolobium contortisiliquum (EcTI) and of its complex with bovine trypsin.PLoS One. 2013 Apr 23;8(4):e62252. doi: 10.1371/journal.pone.0062252. Print 2013. PLoS One. 2013. PMID: 23626794 Free PMC article.
-
Focal adhesion kinase overexpression and its impact on human osteosarcoma.Oncotarget. 2015 Oct 13;6(31):31085-103. doi: 10.18632/oncotarget.5044. Oncotarget. 2015. PMID: 26393679 Free PMC article.
-
Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy.Front Pharmacol. 2016 Dec 8;7:470. doi: 10.3389/fphar.2016.00470. eCollection 2016. Front Pharmacol. 2016. PMID: 28008315 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

