Engineering, expression, purification, and characterization of stable clade A/B recombinant soluble heterotrimeric gp140 proteins
- PMID: 22031951
- PMCID: PMC3255899
- DOI: 10.1128/JVI.06363-11
Engineering, expression, purification, and characterization of stable clade A/B recombinant soluble heterotrimeric gp140 proteins
Abstract
The envelope glycoprotein (Env) of human immunodeficiency virus type 1 (HIV-1) is composed of two noncovalently associated subunits: an extracellular subunit (gp120) and a transmembrane subunit (gp41). The functional unit of Env on the surface of infectious virions is a trimer of gp120/gp41 heterodimers. Env is the target of anti-HIV neutralizing antibodies. A considerable effort has been invested in the engineering of recombinant soluble forms of the virion-associated Env trimer as vaccine candidates to elicit anti-HIV neutralizing antibody responses. These soluble constructs contain three gp120 subunits and the extracellular segments of the corresponding gp41 subunits. The individual gp120/gp41 protomers on these soluble trimers are identical in amino acid sequence (homotrimers). Here, we engineered novel soluble trimeric gp140 proteins that are formed by the association of gp140 protomers that differ in amino acid sequence and glycosylation patterns (heterotrimers). Specifically, we engineered soluble heterotrimeric proteins composed of clade A and clade B Env protomers. The clade A gp140 protomers were derived from viruses isolated during acute infection (Q168a2, Q259d2.17, and Q461e2), whereas the clade B gp140 protomers were derived from a virus isolated during chronic infection (SF162). The amino acid sequence divergence between the clade A and the clade B Envs is approximately 24%. Neutralization epitopes in the CD4 binding sites and coreceptor binding sites, as well as the membrane-proximal external region (MPER), were differentially expressed on the heterotrimeric and homotrimeric proteins. The heterotrimeric gp140s elicited broader anti-tier 1 isolate neutralizing antibody responses than did the homotrimeric gp140s.
Figures
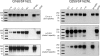




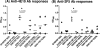
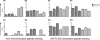

Similar articles
-
Improving the Expression and Purification of Soluble, Recombinant Native-Like HIV-1 Envelope Glycoprotein Trimers by Targeted Sequence Changes.J Virol. 2017 May 26;91(12):e00264-17. doi: 10.1128/JVI.00264-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381572 Free PMC article.
-
Influences on the Design and Purification of Soluble, Recombinant Native-Like HIV-1 Envelope Glycoprotein Trimers.J Virol. 2015 Dec;89(23):12189-210. doi: 10.1128/JVI.01768-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311893 Free PMC article.
-
Single-Chain Soluble BG505.SOSIP gp140 Trimers as Structural and Antigenic Mimics of Mature Closed HIV-1 Env.J Virol. 2015 May;89(10):5318-29. doi: 10.1128/JVI.03451-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740988 Free PMC article.
-
Envelope proteins of two HIV-1 clades induced different epitope-specific antibody response.Vaccine. 2018 Mar 14;36(12):1627-1636. doi: 10.1016/j.vaccine.2018.01.081. Epub 2018 Feb 9. Vaccine. 2018. PMID: 29429810
-
HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation.J Mol Biol. 2011 Jul 22;410(4):582-608. doi: 10.1016/j.jmb.2011.04.042. J Mol Biol. 2011. PMID: 21762802 Free PMC article. Review.
Cited by
-
Characterization of humoral responses to soluble trimeric HIV gp140 from a clade A Ugandan field isolate.J Transl Med. 2013 Jul 8;11:165. doi: 10.1186/1479-5876-11-165. J Transl Med. 2013. PMID: 23835244 Free PMC article.
-
Purification of recombinant vaccinia virus-expressed monomeric HIV-1 gp120 to apparent homogeneity.Protein Expr Purif. 2013 Jul;90(1):34-9. doi: 10.1016/j.pep.2013.04.009. Epub 2013 May 9. Protein Expr Purif. 2013. PMID: 23665667 Free PMC article.
-
Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs.PLoS Pathog. 2013 Jan;9(1):e1003106. doi: 10.1371/journal.ppat.1003106. Epub 2013 Jan 3. PLoS Pathog. 2013. PMID: 23300456 Free PMC article.
-
Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies.AIDS Res Ther. 2017 Sep 12;14(1):50. doi: 10.1186/s12981-017-0178-3. AIDS Res Ther. 2017. PMID: 28893278 Free PMC article. Review.
-
Antigenicity and immunogenicity of a trimeric envelope protein from an Indian clade C HIV-1 isolate.J Biol Chem. 2015 Apr 3;290(14):9195-208. doi: 10.1074/jbc.M114.621185. Epub 2015 Feb 17. J Biol Chem. 2015. PMID: 25691567 Free PMC article.
References
-
- Baba TW, et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 - PubMed
-
- Backliwal G, Hildinger M, Hasija V, Wurm FM. 2008. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol. Bioeng 99:721–727 - PubMed
-
- Beddows S, et al. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329–340 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

