Impaired β-amyloid secretion in Alzheimer's disease pathogenesis
- PMID: 22031884
- PMCID: PMC3225957
- DOI: 10.1523/JNEUROSCI.2986-11.2011
Impaired β-amyloid secretion in Alzheimer's disease pathogenesis
Abstract
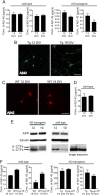
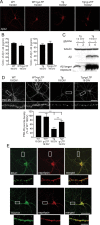
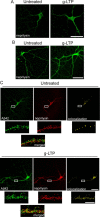
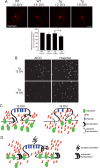
A central question in Alzheimer's disease (AD) research is what role β-amyloid peptide (Aβ) plays in synaptic dysfunction. Synaptic activity increases Aβ secretion, potentially inhibiting synapses, but also decreases intraneuronal Aβ, protecting synapses. We now show that levels of secreted Aβ fall with time in culture in neurons of AD-transgenic mice, but not wild-type mice. Moreover, the ability of synaptic activity to elevate secreted Aβ and reduce intraneuronal Aβ becomes impaired in AD-transgenic but not wild-type neurons with time in culture. We demonstrate that synaptic activity promotes an increase in the Aβ-degrading protease neprilysin at the cell surface and a concomitant increase in colocalization with Aβ42. Remarkably, AD-transgenic but not wild-type neurons show reduced levels of neprilysin with time in culture. This impaired ability to secrete Aβ and reduce intraneuronal Aβ has important implications for the pathogenesis and treatment of AD.
Figures
Similar articles
-
Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations.J Neurosci. 2009 Aug 5;29(31):9704-13. doi: 10.1523/JNEUROSCI.2292-09.2009. J Neurosci. 2009. PMID: 19657023 Free PMC article.
-
Dysregulated phosphorylation of Ca(2+) /calmodulin-dependent protein kinase II-α in the hippocampus of subjects with mild cognitive impairment and Alzheimer's disease.J Neurochem. 2011 Nov;119(4):791-804. doi: 10.1111/j.1471-4159.2011.07447.x. Epub 2011 Sep 28. J Neurochem. 2011. PMID: 21883216 Free PMC article.
-
Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins.FEBS J. 2010 Jul;277(14):3051-67. doi: 10.1111/j.1742-4658.2010.07719.x. Epub 2010 Jun 22. FEBS J. 2010. PMID: 20573181 Free PMC article.
-
Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum?Trends Neurosci. 2001 Apr;24(4):219-24. doi: 10.1016/s0166-2236(00)01749-5. Trends Neurosci. 2001. PMID: 11250006 Review.
-
Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer's disease.Pathol Int. 2017 Apr;67(4):185-193. doi: 10.1111/pin.12520. Epub 2017 Mar 5. Pathol Int. 2017. PMID: 28261941 Review.
Cited by
-
Contribution of Neurons and Glial Cells to Complement-Mediated Synapse Removal during Development, Aging and in Alzheimer's Disease.Mediators Inflamm. 2018 Nov 11;2018:2530414. doi: 10.1155/2018/2530414. eCollection 2018. Mediators Inflamm. 2018. PMID: 30533998 Free PMC article. Review.
-
Alzheimer's disease: relevant molecular and physiopathological events affecting amyloid-β brain balance and the putative role of PPARs.Front Aging Neurosci. 2014 Jul 28;6:176. doi: 10.3389/fnagi.2014.00176. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25120477 Free PMC article. Review.
-
Intracellular distribution of amyloid beta peptide and its relationship to the lysosomal system.Transl Neurodegener. 2012 Sep 26;1(1):19. doi: 10.1186/2047-9158-1-19. Transl Neurodegener. 2012. PMID: 23210724 Free PMC article.
-
Synaptic activity protects against AD and FTD-like pathology via autophagic-lysosomal degradation.Mol Psychiatry. 2018 Jun;23(6):1530-1540. doi: 10.1038/mp.2017.142. Epub 2017 Jul 11. Mol Psychiatry. 2018. PMID: 28696431 Free PMC article.
-
Dual roles for autophagy: degradation and secretion of Alzheimer's disease Aβ peptide.Bioessays. 2014 Jun;36(6):570-8. doi: 10.1002/bies.201400002. Epub 2014 Apr 8. Bioessays. 2014. PMID: 24711225 Free PMC article. Review.
References
-
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging. 2004;25:1263–1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
