Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice
- PMID: 22031018
- PMCID: PMC4533854
- DOI: 10.1002/hep.24764
Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice
Abstract
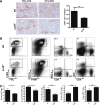
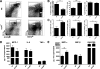
Chemokines critically control the infiltration of immune cells upon liver injury, thereby promoting hepatic inflammation and fibrosis. The chemokine receptor CCR8 can affect trafficking of monocytes/macrophages, monocyte-derived dendritic cells (DCs) and T-helper cell (Th) subsets, but its role in liver diseases is currently unknown. To investigate the functional role of CCR8 in liver diseases, ccr8(-/-) and wild-type (WT) mice were subjected to chronic experimental injury models of carbon tetrachloride (CCl(4) ) administration and surgical bile duct ligation (BDL). CCR8 was strongly up-regulated in the injured liver. Ccr8(-/-) mice displayed attenuated liver damage (e.g., ALT, histology, and TUNEL) compared to WT mice and were also protected from liver fibrosis in two independent injury models. Flow cytometry revealed reduced infiltrates of liver macrophages, neutrophils and natural killer cells, whereas hepatic CD4(+) T cells increased. The main CCR8-expressing cells in the liver were hepatic macrophages, and CCR8 was functionally necessary for CCL1-directed migration of inflammatory but not for nonclassical monocytes into the liver. Moreover, the phenotype of liver macrophages from injured ccr8(-/-) animals was altered with increased expression of DC markers and enhanced expression of T-cell-attracting chemokine macrophage inflammatory protein 1-alpha (MIP-1α/CCL3). Correspondingly, hepatic CD4(+) T cells showed increased Th1 polarization and reduced Th2 cells in CCR8-deficient animals. Liver fibrosis progression, but also subsequent T-cell alterations, could be restored by adoptively transferring CCR8-expressing monocytes/macrophages into ccr8(-/-) mice during experimental injury.
Conclusions: CCR8 critically mediates hepatic macrophage recruitment upon injury, which subsequently shapes the inflammatory response in the injured liver, affecting macrophage/DC and Th differentiation. CCR8 deficiency protects the liver against injury, ameliorating initial inflammatory responses and hepatic fibrogenesis. Inhibition of CCR8 or its ligand, CCL1, might represent a successful therapeutic target to limit liver inflammation and fibrosis progression.
Copyright © 2011 American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures

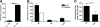
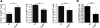
Similar articles
-
The fractalkine receptor CX₃CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes.Hepatology. 2010 Nov;52(5):1769-82. doi: 10.1002/hep.23894. Hepatology. 2010. PMID: 21038415
-
Chemokine receptor CCR8 is required for lipopolysaccharide-triggered cytokine production in mouse peritoneal macrophages.PLoS One. 2014 Apr 8;9(4):e94445. doi: 10.1371/journal.pone.0094445. eCollection 2014. PLoS One. 2014. PMID: 24714157 Free PMC article.
-
Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis.Hepatology. 2014 Feb;59(2):630-42. doi: 10.1002/hep.26697. Epub 2013 Dec 23. Hepatology. 2014. PMID: 23959575 Free PMC article.
-
Macrophage heterogeneity in liver injury and fibrosis.J Hepatol. 2014 May;60(5):1090-6. doi: 10.1016/j.jhep.2013.12.025. Epub 2014 Jan 8. J Hepatol. 2014. PMID: 24412603 Review.
-
Monocytes and macrophages as cellular targets in liver fibrosis.Inflamm Allergy Drug Targets. 2009 Sep;8(4):307-18. doi: 10.2174/187152809789352230. Inflamm Allergy Drug Targets. 2009. PMID: 19534673 Review.
Cited by
-
Differential recruitment of monocyte-derived macrophages in control and stellate cell-depleted mice during recurrent carbon tetrachloride-induced acute liver injury.J Cell Physiol. 2022 Nov;237(11):4215-4225. doi: 10.1002/jcp.30877. Epub 2022 Sep 13. J Cell Physiol. 2022. PMID: 36098042 Free PMC article.
-
Immune mechanisms in acetaminophen-induced acute liver failure.Hepatobiliary Surg Nutr. 2014 Dec;3(6):331-43. doi: 10.3978/j.issn.2304-3881.2014.11.01. Hepatobiliary Surg Nutr. 2014. PMID: 25568858 Free PMC article. Review.
-
Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo.Fibrogenesis Tissue Repair. 2012 Jun 6;5(Suppl 1):S27. doi: 10.1186/1755-1536-5-S1-S27. eCollection 2012. Fibrogenesis Tissue Repair. 2012. PMID: 23259611 Free PMC article.
-
Accumulation of myeloid lineage cells is mapping out liver fibrosis post injury: a targetable lesion using Ketanserin.Exp Mol Med. 2018 Jul 19;50(7):1-13. doi: 10.1038/s12276-018-0118-x. Exp Mol Med. 2018. PMID: 30026607 Free PMC article.
-
Macrophage Function in the Pathogenesis of Non-alcoholic Fatty Liver Disease: The Mac Attack.Front Immunol. 2019 Dec 12;10:2893. doi: 10.3389/fimmu.2019.02893. eCollection 2019. Front Immunol. 2019. PMID: 31921154 Free PMC article. Review.
References
-
- Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008;2:233–242. - PubMed
-
- Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
