Evaluation of the ability of LL-37 to neutralise LPS in vitro and ex vivo
- PMID: 22028895
- PMCID: PMC3196584
- DOI: 10.1371/journal.pone.0026525
Evaluation of the ability of LL-37 to neutralise LPS in vitro and ex vivo
Abstract
Background: Human cathelicidin LL-37 is a cationic antimicrobial peptide (AMP) which possesses a variety of activities including the ability to neutralise endotoxin. In this study, we investigated the role of LPS neutralisation in mediating LL-37's ability to inhibit Pseudomonas aeruginosa LPS signalling in human monocytic cells.
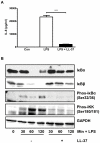
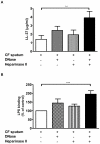
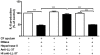
Methodology/principal findings: Pre-treatment of monocytes with LL-37 significantly inhibited LPS-induced IL-8 production and the signalling pathway of associated transcription factors such as NF-κB. However, upon removal of LL-37 from the media prior to LPS stimulation, these inhibitory effects were abolished. These findings suggest that the ability of LL-37 to inhibit LPS signalling is largely dependent on extracellular LPS neutralisation. In addition, LL-37 potently inhibited cytokine production induced by LPS extracted from P. aeruginosa isolated from the lungs of cystic fibrosis (CF) patients. In the CF lung, polyanionic molecules such as glycosaminoglycans (GAGs) and DNA bind LL-37 and impact negatively on its antibacterial activity. In order to determine whether such interactions interfere with the LPS neutralising ability of LL-37, the status of LL-37 and its ability to bind LPS in CF sputum were investigated. Overall our findings suggest that in the CF lung, the ability of LL-37 to bind LPS and inhibit LPS-induced IL-8 production is attenuated as a result of binding to DNA and GAGs. However, LL-37 levels and its concomitant LPS-binding activity can be increased with a combination of DNase and GAG lyase (heparinase II) treatment.
Conclusions/significance: Overall, these findings suggest that a deficiency in available LL-37 in the CF lung may contribute to greater LPS-induced inflammation during CF lung disease.
Conflict of interest statement
Figures
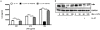

Similar articles
-
Release of the antimicrobial peptide LL-37 from DNA/F-actin bundles in cystic fibrosis sputum.Eur Respir J. 2007 Apr;29(4):624-32. doi: 10.1183/09031936.00080806. Epub 2007 Jan 10. Eur Respir J. 2007. PMID: 17215317
-
LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline.J Immunol. 2009 Jul 1;183(1):543-51. doi: 10.4049/jimmunol.0803959. J Immunol. 2009. PMID: 19542465
-
Bactericidal activities of cathelicidin LL-37 and select cationic lipids against the hypervirulent Pseudomonas aeruginosa strain LESB58.Antimicrob Agents Chemother. 2015 Jul;59(7):3808-15. doi: 10.1128/AAC.00421-15. Epub 2015 Apr 13. Antimicrob Agents Chemother. 2015. PMID: 25870055 Free PMC article.
-
Cathelicidin LL-37: a multitask antimicrobial peptide.Arch Immunol Ther Exp (Warsz). 2010 Feb;58(1):15-25. doi: 10.1007/s00005-009-0057-2. Epub 2010 Jan 5. Arch Immunol Ther Exp (Warsz). 2010. PMID: 20049649 Review.
-
Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide.Ann Agric Environ Med. 2007;14(1):1-4. Ann Agric Environ Med. 2007. PMID: 17655171 Review.
Cited by
-
Cathelicidins Inhibit Escherichia coli-Induced TLR2 and TLR4 Activation in a Viability-Dependent Manner.J Immunol. 2017 Aug 15;199(4):1418-1428. doi: 10.4049/jimmunol.1602164. Epub 2017 Jul 14. J Immunol. 2017. PMID: 28710255 Free PMC article.
-
Complexation of fungal extracellular nucleic acids by host LL-37 peptide shapes neutrophil response to Candida albicans biofilm.Front Immunol. 2024 Feb 6;15:1295168. doi: 10.3389/fimmu.2024.1295168. eCollection 2024. Front Immunol. 2024. PMID: 38384468 Free PMC article.
-
Cathelicidin-like helminth defence molecules (HDMs): absence of cytotoxic, anti-microbial and anti-protozoan activities imply a specific adaptation to immune modulation.PLoS Negl Trop Dis. 2013 Jul 11;7(7):e2307. doi: 10.1371/journal.pntd.0002307. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23875042 Free PMC article.
-
Functionalization of Bacterial Cellulose with the Antimicrobial Peptide KR-12 via Chimerical Cellulose-Binding Peptides.Int J Mol Sci. 2024 Jan 25;25(3):1462. doi: 10.3390/ijms25031462. Int J Mol Sci. 2024. PMID: 38338739 Free PMC article.
-
Effects of LL-37 on Gingival Fibroblasts: A Role in Periodontal Tissue Remodeling?Vaccines (Basel). 2018 Jul 23;6(3):44. doi: 10.3390/vaccines6030044. Vaccines (Basel). 2018. PMID: 30041453 Free PMC article.
References
-
- Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. - PubMed
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. - PubMed
-
- Döring G, Gulbins E. Cystic fibrosis and innate immunity: how chloride channel mutations provoke lung disease. Cell Microbiol. 2009;11:208–216. - PubMed
-
- Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33:537–542. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

