Aspartate 112 is the selectivity filter of the human voltage-gated proton channel
- PMID: 22020278
- PMCID: PMC3237871
- DOI: 10.1038/nature10557
Aspartate 112 is the selectivity filter of the human voltage-gated proton channel
Abstract
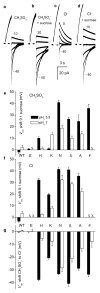
The ion selectivity of pumps and channels is central to their ability to perform a multitude of functions. Here we investigate the mechanism of the extraordinary selectivity of the human voltage-gated proton channel, H(V)1 (also known as HVCN1). This selectivity is essential to its ability to regulate reactive oxygen species production by leukocytes, histamine secretion by basophils, sperm capacitation, and airway pH. The most selective ion channel known, H(V)1 shows no detectable permeability to other ions. Opposing classes of selectivity mechanisms postulate that (1) a titratable amino acid residue in the permeation pathway imparts proton selectivity, or (2) water molecules 'frozen' in a narrow pore conduct protons while excluding other ions. Here we identify aspartate 112 as a crucial component of the selectivity filter of H(V)1. When a neutral amino acid replaced Asp 112, the mutant channel lost proton specificity and became anion-selective or did not conduct. Only the glutamate mutant remained proton-specific. Mutation of the nearby Asp 185 did not impair proton selectivity, indicating that Asp 112 has a unique role. Although histidine shuttles protons in other proteins, when histidine or lysine replaced Asp 112, the mutant channel was still anion-permeable. Evidently, the proton specificity of H(V)1 requires an acidic group at the selectivity filter.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures


Similar articles
-
Peregrination of the selectivity filter delineates the pore of the human voltage-gated proton channel hHV1.J Gen Physiol. 2013 Dec;142(6):625-40. doi: 10.1085/jgp.201311045. Epub 2013 Nov 11. J Gen Physiol. 2013. PMID: 24218398 Free PMC article.
-
Hydrophobic gasket mutation produces gating pore currents in closed human voltage-gated proton channels.Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18951-18961. doi: 10.1073/pnas.1905462116. Epub 2019 Aug 28. Proc Natl Acad Sci U S A. 2019. PMID: 31462498 Free PMC article.
-
A voltage-gated proton-selective channel lacking the pore domain.Nature. 2006 Apr 27;440(7088):1213-6. doi: 10.1038/nature04700. Epub 2006 Mar 22. Nature. 2006. PMID: 16554753 Free PMC article.
-
Voltage-gated proton channels.Cell Mol Life Sci. 2008 Aug;65(16):2554-73. doi: 10.1007/s00018-008-8056-8. Cell Mol Life Sci. 2008. PMID: 18463791 Free PMC article. Review.
-
Voltage-gated proton channels.Compr Physiol. 2012 Apr;2(2):1355-85. doi: 10.1002/cphy.c100071. Compr Physiol. 2012. PMID: 23798303 Free PMC article. Review.
Cited by
-
Voltage-gated proton channels from fungi highlight role of peripheral regions in channel activation.Commun Biol. 2021 Feb 26;4(1):261. doi: 10.1038/s42003-021-01792-0. Commun Biol. 2021. PMID: 33637875 Free PMC article.
-
Engineered high-affinity zinc binding site reveals gating configurations of a human proton channel.J Gen Physiol. 2020 Oct 5;152(10):e202012664. doi: 10.1085/jgp.202012664. J Gen Physiol. 2020. PMID: 32902579 Free PMC article.
-
The intimate and controversial relationship between voltage-gated proton channels and the phagocyte NADPH oxidase.Immunol Rev. 2016 Sep;273(1):194-218. doi: 10.1111/imr.12437. Immunol Rev. 2016. PMID: 27558336 Free PMC article. Review.
-
Structural motifs for subtype-specific pH-sensitive gating of vertebrate otopetrin proton channels.Elife. 2022 Aug 3;11:e77946. doi: 10.7554/eLife.77946. Elife. 2022. PMID: 35920807 Free PMC article.
-
Exotic properties of a voltage-gated proton channel from the snail Helisoma trivolvis.J Gen Physiol. 2018 Jun 4;150(6):835-850. doi: 10.1085/jgp.201711967. Epub 2018 May 9. J Gen Physiol. 2018. PMID: 29743301 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

