Herpes simplex virus requires poly(ADP-ribose) polymerase activity for efficient replication and induces extracellular signal-related kinase-dependent phosphorylation and ICP0-dependent nuclear localization of tankyrase 1
- PMID: 22013039
- PMCID: PMC3255871
- DOI: 10.1128/JVI.05897-11
Herpes simplex virus requires poly(ADP-ribose) polymerase activity for efficient replication and induces extracellular signal-related kinase-dependent phosphorylation and ICP0-dependent nuclear localization of tankyrase 1
Abstract
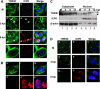
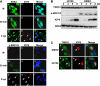
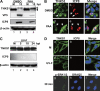
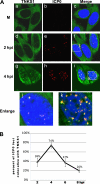
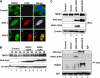
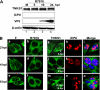
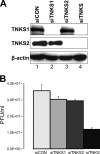
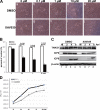
Tankyrase 1 is a poly(ADP-ribose) polymerase (PARP) which localizes to multiple subcellular sites, including telomeres and mitotic centrosomes. Poly(ADP-ribosyl)ation of the nuclear mitotic apparatus (NuMA) protein by tankyrase 1 during mitosis is essential for sister telomere resolution and mitotic spindle pole formation. In interphase cells, tankyrase 1 resides in the cytoplasm, and its role therein is not well understood. In this study, we found that herpes simplex virus (HSV) infection induced extensive modification of tankyrase 1 but not tankyrase 2. This modification was dependent on extracellular signal-regulated kinase (ERK) activity triggered by HSV infection. Following HSV-1 infection, tankyrase 1 was recruited to the nucleus. In the early phase of infection, tankyrase 1 colocalized with ICP0 and thereafter localized within the HSV replication compartment, which was blocked in cells infected with the HSV-1 ICP0-null mutant R7910. In the absence of infection, ICP0 interacted with tankyrase 1 and efficiently promoted its nuclear localization. HSV did not replicate efficiently in cells depleted of both tankyrases 1 and 2. Moreover, XAV939, an inhibitor of tankyrase PARP activity, decreased viral titers to 2 to 5% of control values. We concluded that HSV targets tankyrase 1 in an ICP0- and ERK-dependent manner to facilitate its replication.
Figures

Similar articles
-
Characterization of Elements Regulating the Nuclear-to-Cytoplasmic Translocation of ICP0 in Late Herpes Simplex Virus 1 Infection.J Virol. 2018 Jan 2;92(2):e01673-17. doi: 10.1128/JVI.01673-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093084 Free PMC article.
-
The viral ubiquitin ligase ICP0 is neither sufficient nor necessary for degradation of the cellular DNA sensor IFI16 during herpes simplex virus 1 infection.J Virol. 2013 Dec;87(24):13422-32. doi: 10.1128/JVI.02474-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089555 Free PMC article.
-
NuMA is a major acceptor of poly(ADP-ribosyl)ation by tankyrase 1 in mitosis.Biochem J. 2005 Oct 15;391(Pt 2):177-84. doi: 10.1042/BJ20050885. Biochem J. 2005. PMID: 16076287 Free PMC article.
-
The potential link between PML NBs and ICP0 in regulating lytic and latent infection of HSV-1.Protein Cell. 2012 May;3(5):372-82. doi: 10.1007/s13238-012-2021-x. Epub 2012 Apr 28. Protein Cell. 2012. PMID: 22544561 Free PMC article. Review.
-
Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1.J Virol. 2004 Mar;78(5):2169-78. doi: 10.1128/jvi.78.5.2169-2178.2004. J Virol. 2004. PMID: 14963113 Free PMC article. Review. No abstract available.
Cited by
-
HSV-I and the cellular DNA damage response.Future Virol. 2015 Apr;10(4):383-397. doi: 10.2217/fvl.15.18. Future Virol. 2015. PMID: 26213561 Free PMC article.
-
Scaffold hopping approach on the route to selective tankyrase inhibitors.Eur J Med Chem. 2014 Nov 24;87:611-23. doi: 10.1016/j.ejmech.2014.10.007. Epub 2014 Oct 5. Eur J Med Chem. 2014. PMID: 25299683 Free PMC article.
-
The Herpesvirus Nuclear Egress Complex Component, UL31, Can Be Recruited to Sites of DNA Damage Through Poly-ADP Ribose Binding.Sci Rep. 2017 May 15;7(1):1882. doi: 10.1038/s41598-017-02109-0. Sci Rep. 2017. PMID: 28507315 Free PMC article.
-
The PARP family: insights into functional aspects of poly (ADP-ribose) polymerase-1 in cell growth and survival.Cell Prolif. 2016 Aug;49(4):421-37. doi: 10.1111/cpr.12268. Epub 2016 Jun 22. Cell Prolif. 2016. PMID: 27329285 Free PMC article. Review.
-
Chromatin dynamics during lytic infection with herpes simplex virus 1.Viruses. 2013 Jul 16;5(7):1758-86. doi: 10.3390/v5071758. Viruses. 2013. PMID: 23863878 Free PMC article. Review.
References
-
- Boutell C, Everett RD. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596–36602 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

