Arabidopsis actin depolymerizing factor4 modulates the stochastic dynamic behavior of actin filaments in the cortical array of epidermal cells
- PMID: 22010035
- PMCID: PMC3229145
- DOI: 10.1105/tpc.111.090670
Arabidopsis actin depolymerizing factor4 modulates the stochastic dynamic behavior of actin filaments in the cortical array of epidermal cells
Abstract
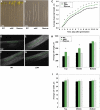
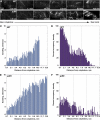
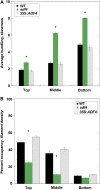
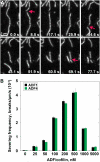
Actin filament arrays are constantly remodeled as the needs of cells change as well as during responses to biotic and abiotic stimuli. Previous studies demonstrate that many single actin filaments in the cortical array of living Arabidopsis thaliana epidermal cells undergo stochastic dynamics, a combination of rapid growth balanced by disassembly from prolific severing activity. Filament turnover and dynamics are well understood from in vitro biochemical analyses and simple reconstituted systems. However, the identification in living cells of the molecular players involved in controlling actin dynamics awaits the use of model systems, especially ones where the power of genetics can be combined with imaging of individual actin filaments at high spatial and temporal resolution. Here, we test the hypothesis that actin depolymerizing factor (ADF)/cofilin contributes to stochastic filament severing and facilitates actin turnover. A knockout mutant for Arabidopsis ADF4 has longer hypocotyls and epidermal cells when compared with wild-type seedlings. This correlates with a change in actin filament architecture; cytoskeletal arrays in adf4 cells are significantly more bundled and less dense than in wild-type cells. Several parameters of single actin filament turnover are also altered. Notably, adf4 mutant cells have a 2.5-fold reduced severing frequency as well as significantly increased actin filament lengths and lifetimes. Thus, we provide evidence that ADF4 contributes to the stochastic dynamic turnover of actin filaments in plant cells.
Figures

Comment in
-
Actin depolymerizing factor4 severs actin filaments in vivo.Plant Cell. 2011 Oct;23(10):3563. doi: 10.1105/tpc.111.231012. Epub 2011 Oct 27. Plant Cell. 2011. PMID: 22034571 Free PMC article. No abstract available.
Similar articles
-
Arabidopsis myosin XI: a motor rules the tracks.Plant Physiol. 2014 Nov;166(3):1359-70. doi: 10.1104/pp.114.244335. Epub 2014 Sep 18. Plant Physiol. 2014. PMID: 25237128 Free PMC article.
-
14-3-3 λ protein interacts with ADF1 to regulate actin cytoskeleton dynamics in Arabidopsis.Sci China Life Sci. 2015 Nov;58(11):1142-50. doi: 10.1007/s11427-015-4897-1. Epub 2015 Aug 7. Sci China Life Sci. 2015. PMID: 26345162
-
ACTIN DEPOLYMERIZING FACTOR4 regulates actin dynamics during innate immune signaling in Arabidopsis.Plant Cell. 2014 Jan;26(1):340-52. doi: 10.1105/tpc.113.122499. Epub 2014 Jan 24. Plant Cell. 2014. PMID: 24464292 Free PMC article.
-
Actin dynamics in the cortical array of plant cells.Curr Opin Plant Biol. 2013 Dec;16(6):678-87. doi: 10.1016/j.pbi.2013.10.012. Epub 2013 Nov 15. Curr Opin Plant Biol. 2013. PMID: 24246228 Review.
-
Functions of actin-interacting protein 1 (AIP1)/WD repeat protein 1 (WDR1) in actin filament dynamics and cytoskeletal regulation.Biochem Biophys Res Commun. 2018 Nov 25;506(2):315-322. doi: 10.1016/j.bbrc.2017.10.096. Epub 2017 Oct 19. Biochem Biophys Res Commun. 2018. PMID: 29056508 Free PMC article. Review.
Cited by
-
Actin depolymerizing factor ADF7 inhibits actin bundling protein VILLIN1 to regulate root hair formation in response to osmotic stress in Arabidopsis.PLoS Genet. 2022 Sep 12;18(9):e1010338. doi: 10.1371/journal.pgen.1010338. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36095000 Free PMC article.
-
TWISTED DWARF1 Mediates the Action of Auxin Transport Inhibitors on Actin Cytoskeleton Dynamics.Plant Cell. 2016 Apr;28(4):930-48. doi: 10.1105/tpc.15.00726. Epub 2016 Apr 6. Plant Cell. 2016. PMID: 27053424 Free PMC article.
-
The Cytoskeleton in Plant Immunity: Dynamics, Regulation, and Function.Int J Mol Sci. 2022 Dec 8;23(24):15553. doi: 10.3390/ijms232415553. Int J Mol Sci. 2022. PMID: 36555194 Free PMC article. Review.
-
CARK3-mediated ADF4 regulates hypocotyl elongation and soil drought stress in Arabidopsis.Front Plant Sci. 2022 Dec 21;13:1065677. doi: 10.3389/fpls.2022.1065677. eCollection 2022. Front Plant Sci. 2022. PMID: 36618656 Free PMC article.
-
Arabidopsis ADF5 Acts as a Downstream Target Gene of CBFs in Response to Low-Temperature Stress.Front Cell Dev Biol. 2021 Jan 28;9:635533. doi: 10.3389/fcell.2021.635533. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33585491 Free PMC article.
References
-
- Andrianantoandro E., Pollard T.D. (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24: 13–23 - PubMed
-
- Augustine R.C., Vidali L., Kleinman K.P., Bezanilla M. (2008). Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J. 54: 863–875 - PubMed
-
- Baskin T.I. (2005). Anisotropic expansion of the plant cell wall. Annu. Rev. Cell Dev. Biol. 21: 203–222 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

