Shared and distinct functions of the transcription factors IRF4 and IRF8 in myeloid cell development
- PMID: 22003407
- PMCID: PMC3189223
- DOI: 10.1371/journal.pone.0025812
Shared and distinct functions of the transcription factors IRF4 and IRF8 in myeloid cell development
Abstract
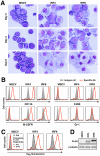
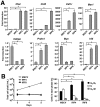
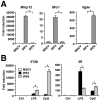
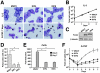
Interferon regulatory factor (IRF) 8 and IRF4 are structurally-related, hematopoietic cell-specific transcription factors that cooperatively regulate the differentiation of dendritic cells and B cells. Whilst in myeloid cells IRF8 is known to modulate growth and differentiation, the role of IRF4 is poorly understood. In this study, we show that IRF4 has activities similar to IRF8 in regulating myeloid cell development. The ectopic expression of IRF4 in myeloid progenitor cells in vitro inhibits cell growth, promotes macrophages, but hinders granulocytic cell differentiation. We also show that IRF4 binds to and activates transcription through the IRF-Ets composite sequence (IECS). Furthermore, we demonstrate that Irf8⁻/⁻Irf4⁻/⁻ mice exhibit a more severe chronic myeloid leukemia (CML)-like disease than Irf8⁻/⁻ mice, involving a disproportionate expansion of granulocytes at the expense of monocytes/macrophages. Irf4⁻/⁻ mice, however, display no obvious abnormality in myeloid cell development, presumably because IRF4 is expressed at a much lower level than IRF8 in granulocyte-macrophage progenitors. Our results also suggest that IRF8 and IRF4 have not only common but also specific activities in myeloid cells. Since the expression of both the IRF8 and IRF4 genes is downregulated in CML patients, these results may add to our understanding of CML pathogenesis.
Conflict of interest statement
Figures


Similar articles
-
Regulation of myelopoiesis by the transcription factor IRF8.Int J Hematol. 2015 Apr;101(4):342-51. doi: 10.1007/s12185-015-1761-9. Epub 2015 Mar 7. Int J Hematol. 2015. PMID: 25749660 Review.
-
IRF8 regulates acid ceramidase expression to mediate apoptosis and suppresses myelogeneous leukemia.Cancer Res. 2011 Apr 15;71(8):2882-91. doi: 10.1158/0008-5472.CAN-10-2493. Epub 2011 Apr 12. Cancer Res. 2011. PMID: 21487040 Free PMC article.
-
Differential expression of IFN regulatory factor 4 gene in human monocyte-derived dendritic cells and macrophages.J Immunol. 2005 Nov 15;175(10):6570-9. doi: 10.4049/jimmunol.175.10.6570. J Immunol. 2005. PMID: 16272311
-
The Granulocyte Progenitor Stage Is a Key Target of IRF8-Mediated Regulation of Myeloid-Derived Suppressor Cell Production.J Immunol. 2017 May 15;198(10):4129-4139. doi: 10.4049/jimmunol.1601722. Epub 2017 Mar 29. J Immunol. 2017. PMID: 28356386 Free PMC article.
-
Transcriptional and Epigenetic Regulation of Innate Immune Cell Development by the Transcription Factor, Interferon Regulatory Factor-8.J Interferon Cytokine Res. 2016 Jul;36(7):433-41. doi: 10.1089/jir.2015.0138. J Interferon Cytokine Res. 2016. PMID: 27379865 Review.
Cited by
-
IRF4 promotes cell proliferation by JNK pathway in multiple myeloma.Med Oncol. 2013;30(2):594. doi: 10.1007/s12032-013-0594-8. Epub 2013 May 12. Med Oncol. 2013. PMID: 23666852
-
Regulating IRFs in IFN Driven Disease.Front Immunol. 2019 Mar 29;10:325. doi: 10.3389/fimmu.2019.00325. eCollection 2019. Front Immunol. 2019. PMID: 30984161 Free PMC article. Review.
-
Regulation of myelopoiesis by the transcription factor IRF8.Int J Hematol. 2015 Apr;101(4):342-51. doi: 10.1007/s12185-015-1761-9. Epub 2015 Mar 7. Int J Hematol. 2015. PMID: 25749660 Review.
-
Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance.Nat Immunol. 2016 May;17(5):545-55. doi: 10.1038/ni.3408. Epub 2016 Mar 28. Nat Immunol. 2016. PMID: 27019226 Free PMC article.
-
Macrophages polarization in renal inflammation and fibrosis animal models (Review).Mol Med Rep. 2024 Feb;29(2):29. doi: 10.3892/mmr.2023.13152. Epub 2023 Dec 22. Mol Med Rep. 2024. PMID: 38131228 Free PMC article. Review.
References
-
- Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. - PubMed
-
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. - PubMed
-
- Rosenbauer F, Tenen DG. Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol. 2007;7:105–117. - PubMed
-
- Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. - PubMed
-
- Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

