The management of schizophrenia: focus on extended-release quetiapine fumarate
- PMID: 22003295
- PMCID: PMC3191868
- DOI: 10.2147/NDT.S3380
The management of schizophrenia: focus on extended-release quetiapine fumarate
Abstract
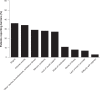
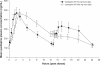
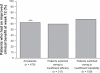
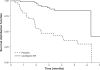
Effective management of schizophrenia remains a significant clinical challenge. While antipsychotic medications have proven efficacy in this disease, there remains an opportunity to further improve symptom control and long-term relapse prevention. Also, a number of factors, including tolerability and complex dosing regimens, can result in nonadherence to medication. Quetiapine is an atypical antipsychotic with proven efficacy and an established tolerability profile in schizophrenia. The once-daily extended-release formulation (quetiapine XR) offers a simplified dosing regimen and titration schedule. Short-term clinical studies have shown that quetiapine XR (400-800 mg/d) is efficacious in the acute treatment of schizophrenia, while a long-term study has shown that quetiapine XR was significantly more effective than placebo at preventing relapse. Furthermore, an investigation in which stable patients switched from the immediate-release formulation (quetiapine IR) to quetiapine XR showed that quetiapine XR is generally well tolerated and has no loss of efficacy compared with quetiapine IR. In patients who experienced insufficient efficacy or poor tolerability on their previous antipsychotic, switching to quetiapine XR significantly improved efficacy compared with the previous treatment. In conclusion, quetiapine XR is an effective and generally well tolerated treatment for schizophrenia. Furthermore, once-daily dosing may improve patient adherence, which may impact positively on patient outcomes.
Keywords: adherence; adverse events; atypical antipsychotics.
Figures

Similar articles
-
The efficacy and safety of once-daily quetiapine extended release in patients with schizophrenia switched from other antipsychotics: an open-label study in Chinese population.BMC Psychiatry. 2015 Jan 22;15:1. doi: 10.1186/s12888-014-0378-5. BMC Psychiatry. 2015. PMID: 25609320 Free PMC article.
-
Update on extended release quetiapine fumarate in schizophrenia and bipolar disorders.Neuropsychiatr Dis Treat. 2012;8:523-36. doi: 10.2147/NDT.S14369. Epub 2012 Nov 8. Neuropsychiatr Dis Treat. 2012. PMID: 23152684 Free PMC article.
-
[Administration of once-daily extended release quetiapine in schizophrenic disorders].Neuropsychopharmacol Hung. 2007 Dec;9(4):189-95. Neuropsychopharmacol Hung. 2007. PMID: 18510263 Clinical Trial. Hungarian.
-
Quetiapine extended release: in schizophrenia.CNS Drugs. 2009;23(3):261-9. doi: 10.2165/00023210-200923030-00007. CNS Drugs. 2009. PMID: 19320534 Review.
-
Pharmacokinetic profile of the extended-release formulation of quetiapine fumarate (quetiapine XR): clinical implications.Curr Med Res Opin. 2013 Jul;29(7):813-25. doi: 10.1185/03007995.2013.794774. Epub 2013 Apr 30. Curr Med Res Opin. 2013. PMID: 23574265 Review.
Cited by
-
The efficacy and safety of once-daily quetiapine extended release in patients with schizophrenia switched from other antipsychotics: an open-label study in Chinese population.BMC Psychiatry. 2015 Jan 22;15:1. doi: 10.1186/s12888-014-0378-5. BMC Psychiatry. 2015. PMID: 25609320 Free PMC article.
-
Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders.Front Psychiatry. 2013 Sep 12;4:102. doi: 10.3389/fpsyt.2013.00102. Front Psychiatry. 2013. PMID: 24062697 Free PMC article. Review.
-
Bioequivalence of two quetiapine extended release tablets in Chinese healthy volunteers under fasting and fed conditions and effects of food on pharmacokinetic profiles.Drug Des Devel Ther. 2018 Dec 31;13:255-264. doi: 10.2147/DDDT.S182965. eCollection 2019. Drug Des Devel Ther. 2018. PMID: 30643391 Free PMC article. Clinical Trial.
-
The dilemma of polypharmacy in psychosis: is it worth combining partial and full dopamine modulation?Int Clin Psychopharmacol. 2022 Nov 1;37(6):263-275. doi: 10.1097/YIC.0000000000000417. Epub 2022 Jul 12. Int Clin Psychopharmacol. 2022. PMID: 35815937 Free PMC article. Review.
-
Drug-induced gastrointestinal disorders.Frontline Gastroenterol. 2014 Jan;5(1):49-57. doi: 10.1136/flgastro-2013-100316. Epub 2013 Jun 19. Frontline Gastroenterol. 2014. PMID: 28839751 Free PMC article. Review.
References
-
- Weiden PJ, Preskorn SH, Fahnestock PA, Carpenter D, Ross R, Docherty JP. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a Roadmap. J Clin Psychiatry. 2007;68(Suppl 7):1–48. - PubMed
-
- Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. - PubMed
-
- Valenstein M, Ganoczy D, McCarthy JF, Myra KH, Lee TA, Blow FC. Antipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective review. J Clin Psychiatry. 2006;67(10):1542–1550. - PubMed
-
- Velligan DI, Lam F, Ereshefsky L, Miller AL. Psychopharmacology: perspectives on medication adherence and atypical antipsychotic medications. Psychiatr Serv. 2003;54(5):665–667. - PubMed
-
- Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437–452. - PubMed
LinkOut - more resources
Full Text Sources

