Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages
- PMID: 21998409
- PMCID: PMC3269210
- DOI: 10.1126/scitranslmed.3003045
Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages
Abstract
Control of tuberculosis worldwide depends on our understanding of human immune mechanisms, which combat the infection. Acquired T cell responses are critical for host defense against microbial pathogens, yet the mechanisms by which they act in humans remain unclear. We report that T cells, by the release of interferon-γ (IFN-γ), induce autophagy, phagosomal maturation, the production of antimicrobial peptides such as cathelicidin, and antimicrobial activity against Mycobacterium tuberculosis in human macrophages via a vitamin D-dependent pathway. IFN-γ induced the antimicrobial pathway in human macrophages cultured in vitamin D-sufficient sera, but not in sera from African-Americans that have lower amounts of vitamin D and who are more susceptible to tuberculosis. In vitro supplementation of vitamin D-deficient serum with 25-hydroxyvitamin D3 restored IFN-γ-induced antimicrobial peptide expression, autophagy, phagosome-lysosome fusion, and antimicrobial activity. These results suggest a mechanism in which vitamin D is required for acquired immunity to overcome the ability of intracellular pathogens to evade macrophage-mediated antimicrobial responses. The present findings underscore the importance of adequate amounts of vitamin D in all human populations for sustaining both innate and acquired immunity against infection.
Figures

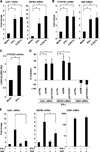
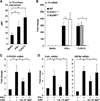
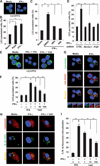
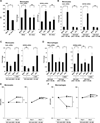
Comment in
-
Infectious disease: a ray of sunshine for TB treatment.Nat Rev Immunol. 2011 Nov 4;11(12):802. doi: 10.1038/nri3110. Nat Rev Immunol. 2011. PMID: 22051891 No abstract available.
Similar articles
-
CD40 ligand and interferon-γ induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes.Immunology. 2013 May;139(1):121-8. doi: 10.1111/imm.12062. Immunology. 2013. PMID: 23289765 Free PMC article. Clinical Trial.
-
Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin.Cell Host Microbe. 2009 Sep 17;6(3):231-43. doi: 10.1016/j.chom.2009.08.004. Cell Host Microbe. 2009. PMID: 19748465
-
Imatinib Triggers Phagolysosome Acidification and Antimicrobial Activity against Mycobacterium bovis Bacille Calmette-Guérin in Glucocorticoid-Treated Human Macrophages.J Immunol. 2016 Jul 1;197(1):222-32. doi: 10.4049/jimmunol.1502407. Epub 2016 May 27. J Immunol. 2016. PMID: 27233968 Free PMC article.
-
Human macrophage host defense against Mycobacterium tuberculosis.Curr Opin Immunol. 2008 Aug;20(4):371-6. doi: 10.1016/j.coi.2008.05.014. Epub 2008 Jul 21. Curr Opin Immunol. 2008. PMID: 18602003 Review.
-
New insights into the interaction of Mycobacterium tuberculosis and human macrophages.Future Microbiol. 2014;9(3):327-41. doi: 10.2217/fmb.13.164. Future Microbiol. 2014. PMID: 24762307 Review.
Cited by
-
Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome.Clin Infect Dis. 2012 Oct;55(7):1004-11. doi: 10.1093/cid/cis577. Epub 2012 Jun 19. Clin Infect Dis. 2012. PMID: 22715179 Free PMC article.
-
The nonskeletal effects of vitamin D: an Endocrine Society scientific statement.Endocr Rev. 2012 Jun;33(3):456-92. doi: 10.1210/er.2012-1000. Epub 2012 May 17. Endocr Rev. 2012. PMID: 22596255 Free PMC article. Review.
-
Autophagy and intestinal homeostasis.Annu Rev Physiol. 2013;75:241-62. doi: 10.1146/annurev-physiol-030212-183658. Epub 2012 Dec 3. Annu Rev Physiol. 2013. PMID: 23216414 Free PMC article. Review.
-
Effect of vitamin D supplementation on Mycobacterium tuberculosis-induced innate immune responses in a Canadian Dené First Nations cohort.PLoS One. 2012;7(7):e40692. doi: 10.1371/journal.pone.0040692. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22866178 Free PMC article.
-
MIR144* inhibits antimicrobial responses against Mycobacterium tuberculosis in human monocytes and macrophages by targeting the autophagy protein DRAM2.Autophagy. 2017 Feb;13(2):423-441. doi: 10.1080/15548627.2016.1241922. Epub 2016 Oct 20. Autophagy. 2017. PMID: 27764573 Free PMC article.
References
-
- World Health Organization. Geneva: World Health Organization; 2009. Tuberculosis Facts, 2009 Update. http://www.who.int/tb/publications/2009/factsheet_tb_2009update_dec09.pdf.
-
- Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 1999;340:367–373. - PubMed
-
- Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-γ–receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 1996;335:1941–1949. - PubMed
-
- Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, Döffinger R, Altare F, Girdlestone J, Emile JF, Ducoulombier H, Edgar D, Clarke J, Oxelius VA, Brai M, Novelli V, Heyne K, Fischer A, Holland SM, Kumararatne DS, Schreiber RD, Casanova JL. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat. Genet. 1999;21:370–378. - PubMed
-
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR033176/RR/NCRR NIH HHS/United States
- R01 AR040312-21/AR/NIAMS NIH HHS/United States
- R01 AI047868-11A1/AI/NIAID NIH HHS/United States
- R01 AI022553-27/AI/NIAID NIH HHS/United States
- R01 AI047868/AI/NIAID NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- AI073539/AI/NIAID NIH HHS/United States
- R01 AR040312/AR/NIAMS NIH HHS/United States
- AI047868/AI/NIAID NIH HHS/United States
- R01 AI073539/AI/NIAID NIH HHS/United States
- AR040312/AR/NIAMS NIH HHS/United States
- R01 AI022553/AI/NIAID NIH HHS/United States
- AI022553/AI/NIAID NIH HHS/United States
- R37 AI047868/AI/NIAID NIH HHS/United States
- R01 AI073539-03/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

