Functional genomics reveals an essential and specific role for Stat1 in protection of the central nervous system following herpes simplex virus corneal infection
- PMID: 21994441
- PMCID: PMC3233176
- DOI: 10.1128/JVI.06032-11
Functional genomics reveals an essential and specific role for Stat1 in protection of the central nervous system following herpes simplex virus corneal infection
Abstract
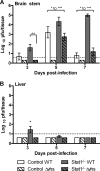
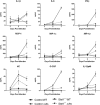
Innate immune deficiencies result in a spectrum of severe clinical outcomes following infection. In particular, there is a strong association between loss of the signal transducer and activator of transcription (Stat) pathway, breach of the blood-brain barrier (BBB), and virus-induced neuropathology. The gene signatures that characterize resistance, disease, and mortality in the virus-infected nervous system have not been defined. Herpes simplex virus type 1 (HSV-1) is commonly associated with encephalitis in humans, and humans and mice lacking Stat1 display increased susceptibility to HSV central nervous system (CNS) infections. In this study, two HSV-1 strains were used, KOS (wild type [WT]), and Δvhs, an avirulent recombinant lacking the virion host shutoff (vhs) function. In addition, two mouse strains were used: strain 129 (control) and a Stat1-deficient (Stat1(-/-)) strain. Using combinations of these virus and mouse strains, we established a model of infection resulting in three different outcomes: viral clearance without neurological disease (Δvhs infection of control mice), neurological disease followed by viral clearance (Δvhs infection of Stat1(-/-) mice and WT infection of control mice), or neurological disease followed by death (WT infection of Stat1(-/-) mice). Through the use of functional genomics on the infected brain stems, we determined gene signatures that were representative of the three infection outcomes. We demonstrated a pathological signature in the brain stem of Stat1-deficient mice characterized by upregulation of transcripts encoding chemokine receptors, inflammatory markers, neutrophil chemoattractants, leukocyte adhesion proteins, and matrix metalloproteases. Additionally, there was a greater than 100-fold increase in the inflammatory markers interleukin 1β (IL-1β) and IL-6. Consistent with this gene signature, we demonstrated profound CNS inflammation with a concomitant lethal breach of the BBB. Taken together, our results indicated an essential role for normal Stat1-dependent signaling in mediating a nonpathological immune response to viral CNS infection.
Figures




Similar articles
-
Host responses to wild-type and attenuated herpes simplex virus infection in the absence of Stat1.J Virol. 2009 Mar;83(5):2075-87. doi: 10.1128/JVI.02007-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109391 Free PMC article.
-
Immune- and Nonimmune-Compartment-Specific Interferon Responses Are Critical Determinants of Herpes Simplex Virus-Induced Generalized Infections and Acute Liver Failure.J Virol. 2016 Nov 14;90(23):10789-10799. doi: 10.1128/JVI.01473-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681121 Free PMC article.
-
Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice.J Virol. 2000 Apr;74(8):3598-604. doi: 10.1128/jvi.74.8.3598-3604.2000. J Virol. 2000. PMID: 10729135 Free PMC article.
-
Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans.Curr Opin Allergy Clin Immunol. 2007 Dec;7(6):495-505. doi: 10.1097/ACI.0b013e3282f151d2. Curr Opin Allergy Clin Immunol. 2007. PMID: 17989525 Review.
-
Microglial response to viral challenges: every silver lining comes with a cloud.Front Biosci (Landmark Ed). 2011 Jun 1;16(6):2187-205. doi: 10.2741/3847. Front Biosci (Landmark Ed). 2011. PMID: 21622170 Review.
Cited by
-
The Type I Interferon Response Determines Differences in Choroid Plexus Susceptibility between Newborns and Adults in Herpes Simplex Virus Encephalitis.mBio. 2016 Apr 12;7(2):e00437-16. doi: 10.1128/mBio.00437-16. mBio. 2016. PMID: 27073094 Free PMC article.
-
Genome-wide mouse mutagenesis reveals CD45-mediated T cell function as critical in protective immunity to HSV-1.PLoS Pathog. 2013 Sep;9(9):e1003637. doi: 10.1371/journal.ppat.1003637. Epub 2013 Sep 12. PLoS Pathog. 2013. PMID: 24068938 Free PMC article.
-
PNKP knockdown by RNA interference inhibits herpes simplex virus-1 replication in astrocytes.Virol Sin. 2013 Dec;28(6):345-51. doi: 10.1007/s12250-013-3350-5. Epub 2013 Nov 6. Virol Sin. 2013. PMID: 24213989 Free PMC article.
-
Induction of Multiple miR-200/182 Members in the Brains of Mice Are Associated with Acute Herpes Simplex Virus 1 Encephalitis.PLoS One. 2017 Jan 3;12(1):e0169081. doi: 10.1371/journal.pone.0169081. eCollection 2017. PLoS One. 2017. PMID: 28045967 Free PMC article.
-
Von Willebrand Factor Gene Variants Associate with Herpes simplex Encephalitis.PLoS One. 2016 May 25;11(5):e0155832. doi: 10.1371/journal.pone.0155832. eCollection 2016. PLoS One. 2016. PMID: 27224245 Free PMC article.
References
-
- Aravalli R. N., Hu S., Rowen T. N., Palmquist J. M., Lokensgard J. R. 2005. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 175:4189–4193 - PubMed
-
- Argaw A. T., et al. 2006. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 177:5574–5584 - PubMed
-
- Baringer J. R. 2008. Herpes simplex infections of the nervous system. Neurol. Clin. 26:657–674, viii - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

