Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening
- PMID: 21993836
- PMCID: PMC3719864
- DOI: 10.1039/c1ib00073j
Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening
Abstract
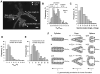
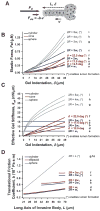
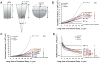
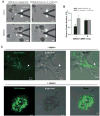
Collective cell invasion (CCI) through interstitial collagenous extracellular matrix (ECM) is crucial to the initial stages of branching morphogenesis, and a hallmark of tissue repair and dissemination of certain tumors. The collagenous ECM acts as a mechanical barrier against CCI. However, the physical nature of this barrier and how it is overcome by cells remains incompletely understood. To address these questions, we performed theoretical and experimental analysis of mammary epithelial branching morphogenesis in 3D type I collagen (collagen-I) gels. We found that the mechanical resistance of collagen-I is largely due to its elastic rather than its viscous properties. We also identified two strategies utilized by mammary epithelial cells that can independently minimize ECM mechanical resistance during CCI. First, cells adopt a narrow tube-like geometry during invasion, which minimizes the elastic opposition from the ECM as revealed by theoretical modeling of the most frequent invasive shapes and sizes. Second, the stiffness of the collagenous ECM is reduced at invasive fronts due to its degradation by matrix metalloproteinases (MMPs), as indicated by direct measurements of collagen-I microelasticity by atomic force microscopy. Molecular techniques further specified that the membrane-bound MMP14 mediates degradation of collagen-I at invasive fronts. Thus, our findings reveal that MMP14 is necessary to efficiently reduce the physical restraints imposed by collagen-I during branching morphogenesis, and help our overall understanding of how forces are balanced between cells and their surrounding ECM to maintain collective geometry and mechanical stability during CCI.
Figures

Similar articles
-
Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin β1.Development. 2013 Jan 15;140(2):343-52. doi: 10.1242/dev.084236. Development. 2013. PMID: 23250208 Free PMC article.
-
Divergent Matrix-Remodeling Strategies Distinguish Developmental from Neoplastic Mammary Epithelial Cell Invasion Programs.Dev Cell. 2018 Oct 22;47(2):145-160.e6. doi: 10.1016/j.devcel.2018.08.025. Epub 2018 Sep 27. Dev Cell. 2018. PMID: 30269950 Free PMC article.
-
Three-dimensional modeling of mechanical forces in the extracellular matrix during epithelial lumen formation.Biophys J. 2006 Jun 15;90(12):4380-91. doi: 10.1529/biophysj.105.073494. Epub 2006 Mar 24. Biophys J. 2006. PMID: 16565042 Free PMC article.
-
Heterotypic control of basement membrane dynamics during branching morphogenesis.Dev Biol. 2015 May 1;401(1):103-9. doi: 10.1016/j.ydbio.2014.12.011. Epub 2014 Dec 16. Dev Biol. 2015. PMID: 25527075 Free PMC article. Review.
-
Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes.Breast Cancer Res. 2004;6(1):1-11. doi: 10.1186/bcr634. Epub 2003 Aug 19. Breast Cancer Res. 2004. PMID: 14680479 Free PMC article. Review.
Cited by
-
Engineered extracellular matrices: emerging strategies for decoupling structural and molecular signals that regulate epithelial branching morphogenesis.Curr Opin Biomed Eng. 2020 Mar;13:103-112. doi: 10.1016/j.cobme.2019.12.013. Epub 2020 Jan 3. Curr Opin Biomed Eng. 2020. PMID: 32864528 Free PMC article.
-
Cellular and physical mechanisms of branching morphogenesis.Development. 2014 Jul;141(14):2750-9. doi: 10.1242/dev.104794. Development. 2014. PMID: 25005470 Free PMC article. Review.
-
Dynamic Expression of Membrane Type 1-Matrix Metalloproteinase (Mt1-mmp/Mmp14) in the Mouse Embryo.Cells. 2021 Sep 17;10(9):2448. doi: 10.3390/cells10092448. Cells. 2021. PMID: 34572097 Free PMC article.
-
Actomyosin contractility requirements and reciprocal cell-tissue mechanics for cancer cell invasion through collagen-based channels.Eur Phys J E Soft Matter. 2022 May 16;45(5):48. doi: 10.1140/epje/s10189-022-00182-6. Eur Phys J E Soft Matter. 2022. PMID: 35575822 Free PMC article.
-
Dual role of E-cadherin in the regulation of invasive collective migration of mammary carcinoma cells.Sci Rep. 2018 Mar 21;8(1):4986. doi: 10.1038/s41598-018-22940-3. Sci Rep. 2018. PMID: 29563585 Free PMC article.
References
-
- Fleury V, Watanabe T, Nguyen T, Unbekandt M, Warburton D, Dejmek M, Nguyen MB, Lindner A, Schwartz L. In: Branching Morphogenesis. Davies JA, editor. Landes Bioscience; Georgetown: 2005. pp. 202–234.
-
- Friedl P, Gilmour D. Nat Rev Mol Cell Biol. 2009;10:445–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

