Porcine vena cava as an alternative to bovine pericardium in bioprosthetic percutaneous heart valves
- PMID: 21993239
- PMCID: PMC3208764
- DOI: 10.1016/j.biomaterials.2011.09.027
Porcine vena cava as an alternative to bovine pericardium in bioprosthetic percutaneous heart valves
Abstract
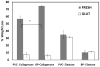
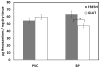
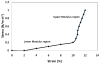
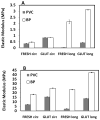
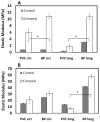
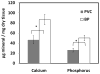
Percutaneous heart valves are revolutionizing valve replacement surgery by offering a less invasive treatment option for high-risk patient populations who have previously been denied the traditional open chest procedure. Percutaneous valves need to be crimped to accommodate a small-diameter catheter during deployment, and they must then open to the size of heart valve. Thus the material used must be strong and possess elastic recoil for this application. Most percutaneous valves utilize bovine pericardium as a material of choice. One possible method to reduce the device delivery diameter is to utilize a thin, highly elastic tissue. Here we investigated porcine vena cava as an alternative to bovine pericardium for percutaneous valve application. We compared the structural, mechanical, and in vivo properties of porcine vena cava to those of bovine pericardium. While the extracellular matrix fibers of pericardium are randomly oriented, the vena cava contains highly aligned collagen and elastin fibers that impart strength to the vessel in the circumferential direction and elasticity in the longitudinal direction. Moreover, the vena cava contains a greater proportion of elastin, whereas the pericardium matrix is mainly composed of collagen. Due to its high elastin content, the vena cava is significantly less stiff than the pericardium, even after crosslinking with glutaraldehyde. Furthermore, the vena cava's mechanical compliance is preserved after compression under forces similar to those exerted by a stent, whereas pericardium is significantly stiffened by this process. Bovine pericardium also showed surface cracks observed by scanning electron microscopy after crimping that were not seen in vena cava tissue. Additionally, the vena cava exhibited reduced calcification (46.64 ± 8.15 μg Ca/mg tissue) as compared to the pericardium (86.79 ± 10.34 μg/mg). These results suggest that the vena cava may provide enhanced leaflet flexibility, tissue resilience, and tissue integrity in percutaneous heart valves, ultimately reducing the device profile while improving the durability of these valves.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Schoen FJ, Levy RJ. Founder’s Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999;47:439–65. - PubMed
-
- Culliford AT, Galloway AC, Colvin SB, Grossi EA, Baumann FG, Esposito R, et al. Aortic valve replacement for aortic stenosis in persons aged 80 years and over. Am J Cardiol. 1991;67:1256–60. - PubMed
-
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35:747–56. - PubMed
-
- Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

