Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice
- PMID: 21982232
- PMCID: PMC3538360
- DOI: 10.1016/j.stem.2011.09.001
Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice
Abstract
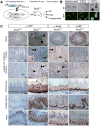
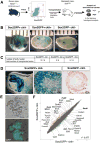
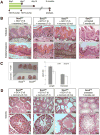
The transcription factor Sox2 maintains the pluripotency of early embryonic cells and regulates the formation of several epithelia during fetal development. Whether Sox2 continues to play a role in adult tissues remains largely unknown. We show here that Sox2 marks adult cells in several epithelial tissues where its expression has not previously been characterized, including the stomach, cervix, anus, testes, lens, and multiple glands. Genetic lineage tracing and transplantation experiments demonstrate that Sox2-expressing cells continuously give rise to mature cell types within these tissues, documenting their self-renewal and differentiation potentials. Consistent with these findings, ablation of Sox2(+) cells in mice results in a disruption of epithelial tissue homeostasis and lethality. Developmental fate mapping reveals that Sox2(+) adult stem cells originate from fetal Sox2(+) tissue progenitors. Thus, our results identify Sox2 expression in numerous adult endodermal and ectodermal stem cell compartments, which are critical for normal tissue regeneration and survival.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Long live sox2: sox2 lasts a lifetime.Cell Stem Cell. 2011 Oct 4;9(4):283-4. doi: 10.1016/j.stem.2011.09.007. Cell Stem Cell. 2011. PMID: 21982223
Similar articles
-
Sox2 Expression Marks Castration-Resistant Progenitor Cells in the Adult Murine Prostate.Stem Cells. 2019 May;37(5):690-700. doi: 10.1002/stem.2987. Epub 2019 Feb 15. Stem Cells. 2019. PMID: 30720908 Free PMC article.
-
In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus.Cell Stem Cell. 2007 Nov;1(5):515-28. doi: 10.1016/j.stem.2007.09.002. Cell Stem Cell. 2007. PMID: 18371391 Free PMC article.
-
Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression.Stem Cells. 2008 Oct;26(10):2467-74. doi: 10.1634/stemcells.2008-0317. Epub 2008 Jul 17. Stem Cells. 2008. PMID: 18635867
-
A plethora of human pluripotent stem cells.Cell Biol Int. 2013 Sep;37(9):875-87. doi: 10.1002/cbin.10120. Epub 2013 May 23. Cell Biol Int. 2013. PMID: 23619972 Review.
-
Tracing the Dynamics of Stem Cell Fate.Cold Spring Harb Perspect Biol. 2020 Jun 1;12(6):a036202. doi: 10.1101/cshperspect.a036202. Cold Spring Harb Perspect Biol. 2020. PMID: 31932319 Free PMC article. Review.
Cited by
-
A localized Wnt signal orients asymmetric stem cell division in vitro.Science. 2013 Mar 22;339(6126):1445-8. doi: 10.1126/science.1231077. Science. 2013. PMID: 23520113 Free PMC article.
-
Pluripotency and Epigenetic Factors in Mouse Embryonic Stem Cell Fate Regulation.Mol Cell Biol. 2015 Aug;35(16):2716-28. doi: 10.1128/MCB.00266-15. Epub 2015 Jun 1. Mol Cell Biol. 2015. PMID: 26031336 Free PMC article. Review.
-
Epithelial Regeneration After Gastric Ulceration Causes Prolonged Cell-Type Alterations.Cell Mol Gastroenterol Hepatol. 2016 May 17;2(5):625-647. doi: 10.1016/j.jcmgh.2016.05.005. eCollection 2016 Sep. Cell Mol Gastroenterol Hepatol. 2016. PMID: 27766298 Free PMC article.
-
Cerebellum lineage allocation, morphogenesis and repair: impact of interplay amongst cells.Development. 2022 Sep 15;149(18):dev185587. doi: 10.1242/dev.185587. Epub 2022 Sep 29. Development. 2022. PMID: 36172987 Free PMC article. Review.
-
Tracing epithelial stem cells during development, homeostasis, and repair.J Cell Biol. 2012 May 28;197(5):575-84. doi: 10.1083/jcb.201201041. J Cell Biol. 2012. PMID: 22641343 Free PMC article. Review.
References
-
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell stem cell. 7:656–670. - PubMed
-
- Barker N, Huch M, Kujala P, van den Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

