Tyrosine phosphorylated c-Cbl regulates platelet functional responses mediated by outside-in signaling
- PMID: 21967979
- PMCID: PMC3217362
- DOI: 10.1182/blood-2011-01-328807
Tyrosine phosphorylated c-Cbl regulates platelet functional responses mediated by outside-in signaling
Abstract
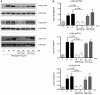
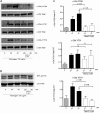
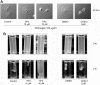
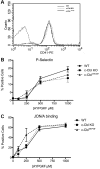
c-Cbl protein functions as an E3 ligase and scaffolding protein, where 3 residues, Y700, Y731, and Y774, upon phosphorylation, have been shown to initiate several signaling cascades. In this study, we investigated the role of these phospho-tyrosine residues in the platelet functional responses after integrin engagement. We observed that c-Cbl Y700, Y731 and Y774 undergo phosphorylation upon platelet adhesion to immobilized fibrinogen, which was inhibited in the presence of PP2, a pan-src family kinase (SFK) inhibitor, suggesting that c-Cbl is phosphorylated downstream of SFKs. However, OXSI-2, a Syk inhibitor, significantly reduced c-Cbl phosphorylation at residues Y774 and Y700, without affecting Y731 phosphorylation. Interestingly, PP2 inhibited both platelet-spreading on fibrinogen as well as clot retraction, whereas OXSI-2 blocked only platelet-spreading, suggesting a differential role of these tyrosine residues. The physiologic role of c-Cbl and Y731 was studied using platelets from c-Cbl KO and c-Cbl(YF/YF) knock-in mice. c-Cbl KO and c-Cbl(YF/YF) platelets had a significantly reduced spreading over immobilized fibrinogen. Furthermore, clot retraction with c-Cbl KO and c-Cbl(YF/YF) platelets was drastically delayed. These results indicate that c-Cbl and particularly its phosphorylated residue Y731 plays an important role in platelet outside-in signaling contributing to platelet-spreading and clot retraction.
Figures


Similar articles
-
Characterization of Integrin αIIbβ3-Mediated Outside-in Signaling by Protein Kinase Cδ in Platelets.Int J Mol Sci. 2020 Sep 8;21(18):6563. doi: 10.3390/ijms21186563. Int J Mol Sci. 2020. PMID: 32911704 Free PMC article.
-
Platelet alpha IIb-beta 3 integrin engagement induces the tyrosine phosphorylation of Cbl and its association with phosphoinositide 3-kinase and Syk.Biochem J. 2000 Nov 1;351 Pt 3(Pt 3):669-76. Biochem J. 2000. PMID: 11042121 Free PMC article.
-
The loss of Cbl-phosphatidylinositol 3-kinase interaction perturbs RANKL-mediated signaling, inhibiting bone resorption and promoting osteoclast survival.J Biol Chem. 2010 Nov 19;285(47):36745-58. doi: 10.1074/jbc.M110.124628. Epub 2010 Sep 17. J Biol Chem. 2010. PMID: 20851882 Free PMC article.
-
The Cbl family proteins: ring leaders in regulation of cell signaling.J Cell Physiol. 2006 Oct;209(1):21-43. doi: 10.1002/jcp.20694. J Cell Physiol. 2006. PMID: 16741904 Review.
-
Platelet Src family kinases: A tale of reversible phosphorylation.Res Pract Thromb Haemost. 2021 Mar 26;5(3):376-389. doi: 10.1002/rth2.12495. eCollection 2021 Mar. Res Pract Thromb Haemost. 2021. PMID: 33870023 Free PMC article. Review.
Cited by
-
Suppression of Grape White Rot Caused by Coniella vitis Using the Potential Biocontrol Agent Bacillus velezensis GSBZ09.Pathogens. 2022 Feb 14;11(2):248. doi: 10.3390/pathogens11020248. Pathogens. 2022. PMID: 35215191 Free PMC article.
-
Dominant role of αIIbβ3 in platelet interactions with cross-linked fibrin fragment D-dimer.Blood Adv. 2020 Jul 14;4(13):2939-2949. doi: 10.1182/bloodadvances.2020001545. Blood Adv. 2020. PMID: 32603423 Free PMC article.
-
Integrin αIIbβ3 outside-in signaling.Blood. 2017 Oct 5;130(14):1607-1619. doi: 10.1182/blood-2017-03-773614. Epub 2017 Aug 9. Blood. 2017. PMID: 28794070 Free PMC article. Review.
-
Acetylation, Phosphorylation, Ubiquitination (Oh My!): Following Post-Translational Modifications on the Ubiquitin Road.Biomolecules. 2022 Mar 18;12(3):467. doi: 10.3390/biom12030467. Biomolecules. 2022. PMID: 35327659 Free PMC article. Review.
-
Arsenic promotes ubiquitinylation and lysosomal degradation of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels in human airway epithelial cells.J Biol Chem. 2012 May 18;287(21):17130-17139. doi: 10.1074/jbc.M111.338855. Epub 2012 Mar 30. J Biol Chem. 2012. PMID: 22467879 Free PMC article.
References
-
- Arias-Salgado EG, Lizano S, Shattil SJ, Ginsberg MH. Specification of the direction of adhesive signaling by the integrin beta cytoplasmic domain. J Biol Chem. 2005;280(33):29699–29707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

