Insulin resistance and metabolic hepatocarcinogenesis with parent-of-origin effects in A×B mice
- PMID: 21967816
- PMCID: PMC3260805
- DOI: 10.1016/j.ajpath.2011.08.014
Insulin resistance and metabolic hepatocarcinogenesis with parent-of-origin effects in A×B mice
Abstract
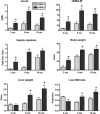
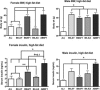
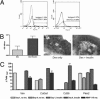
Insulin resistance is a defining feature of metabolic syndrome and type 2 diabetes mellitus but also may occur independently of these conditions. Nonalcoholic fatty liver disease (NAFLD), the hepatic manifestation of these disorders, increases the risk of hepatocellular carcinoma (HCC). However, mechanisms linking hyperinsulinemia to NAFLD and HCC require clarification. We describe a novel model of primary insulin resistance and HCC with strong parent-of-origin effects. Male AB6F1 (A/JCr dam × C57BL/6 sire) but not B6AF1 (B6 dam × A/J sire) mice developed spontaneous insulin resistance, NAFLD, and HCC without obesity or diabetes. A survey of mitochondrial, imprinted, and sex-linked traits revealed modest associations with X-linked genes. However, a diet-induced obesity study, including B6.A chromosome substitution-strain (consomic) mice, showed no segregation by sex chromosome. Thus, parent-of-origin effects were specified within the autosomal genome. Next, we interrogated mechanisms of insulin-associated hepatocarcinogenesis. Steatotic hepatocytes exhibited adipogenic transition characterized by vacuolar metaplasia and up-regulation of vimentin, adipsin, fatty acid translocase (CD36), peroxisome proliferator-activated receptor-γ, and related products. This profile was largely recapitulated in insulin-supplemented primary mouse hepatocyte cultures. Importantly, pyruvate kinase M2, a fetal anabolic enzyme implicated in the Warburg effect, was activated by insulin in vivo and in vitro. Thus, our study reveals parent-of-origin effects in heritable insulin resistance, implicating adipogenic transition with acquired anabolic metabolism in the progression from NAFLD to HCC.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Insulin resistance, steatohepatitis, and hepatocellular carcinoma in a new congenic strain of Fatty Liver Shionogi (FLS) mice with the Lep(ob) gene.Exp Anim. 2010;59(4):407-19. doi: 10.1538/expanim.59.407. Exp Anim. 2010. PMID: 20660987
-
Adipocyte JAK2 mediates spontaneous metabolic liver disease and hepatocellular carcinoma.JCI Insight. 2019 Aug 8;5(17):e131310. doi: 10.1172/jci.insight.131310. JCI Insight. 2019. PMID: 31393852 Free PMC article.
-
Peroxisomal β-oxidation regulates whole body metabolism, inflammatory vigor, and pathogenesis of nonalcoholic fatty liver disease.JCI Insight. 2018 Mar 22;3(6):e93626. doi: 10.1172/jci.insight.93626. JCI Insight. 2018. PMID: 29563328 Free PMC article.
-
Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question.World J Gastroenterol. 2015 Apr 14;21(14):4103-10. doi: 10.3748/wjg.v21.i14.4103. World J Gastroenterol. 2015. PMID: 25892859 Free PMC article. Review.
-
Nonalcoholic fatty liver disease and hepatocellular carcinoma.Metabolism. 2016 Aug;65(8):1151-60. doi: 10.1016/j.metabol.2016.01.010. Epub 2016 Jan 23. Metabolism. 2016. PMID: 26907206 Review.
Cited by
-
Sex-Specific Parental Effects on Offspring Lipid Levels.J Am Heart Assoc. 2015 Jun 30;4(7):e001951. doi: 10.1161/JAHA.115.001951. J Am Heart Assoc. 2015. PMID: 26126546 Free PMC article.
-
Colocalization of MID1IP1 and c-Myc is Critically Involved in Liver Cancer Growth via Regulation of Ribosomal Protein L5 and L11 and CNOT2.Cells. 2020 Apr 16;9(4):985. doi: 10.3390/cells9040985. Cells. 2020. PMID: 32316188 Free PMC article.
-
Silencing vimentin expression decreases pulmonary metastases in a pre-diabetic mouse model of mammary tumor progression.Oncogene. 2017 Mar;36(10):1394-1403. doi: 10.1038/onc.2016.305. Epub 2016 Aug 29. Oncogene. 2017. PMID: 27568979 Free PMC article.
-
Human CYP2B6 produces oxylipins from polyunsaturated fatty acids and reduces diet-induced obesity.PLoS One. 2022 Dec 15;17(12):e0277053. doi: 10.1371/journal.pone.0277053. eCollection 2022. PLoS One. 2022. PMID: 36520866 Free PMC article.
-
Prolactin prevents hepatocellular carcinoma by restricting innate immune activation of c-Myc in mice.Proc Natl Acad Sci U S A. 2014 Aug 5;111(31):11455-60. doi: 10.1073/pnas.1404267111. Epub 2014 Jul 21. Proc Natl Acad Sci U S A. 2014. PMID: 25049387 Free PMC article.
References
-
- James W.P. WHO recognition of the global obesity epidemic. Int J Obes. 2005;32(Suppl):S120–S126. - PubMed
-
- Pender J.R., Pories W.J. Epidemiology of obesity in the United States. Gastroenterol Clin North Am. 2005;34:1–7. - PubMed
-
- Ertle J., Dechene A., Sowa J.P., Penndorf V., Herzer K., Kaiser G., Schlaak J.F., Gerken G., Syn W.K., Canbay A. Nonalcoholic fatty liver disease progresses to HCC in the absence of apparent cirrhosis. Int J Cancer. 2010;128:2436–2443. - PubMed
-
- El-Serag H.B. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl 2):S88–S94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30ES02109/ES/NIEHS NIH HHS/United States
- P01CA0267/CA/NCI NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- CA158661/CA/NCI NIH HHS/United States
- P30 ES002109/ES/NIEHS NIH HHS/United States
- CA067529/CA/NCI NIH HHS/United States
- T32 RR007036/RR/NCRR NIH HHS/United States
- R01 CA067529/CA/NCI NIH HHS/United States
- RR007036/RR/NCRR NIH HHS/United States
- AA016563/AA/NIAAA NIH HHS/United States
- CA016086/CA/NCI NIH HHS/United States
- R21 CA158661/CA/NCI NIH HHS/United States
- K01 AA016563/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

