Task-level feedback can explain temporal recruitment of spatially fixed muscle synergies throughout postural perturbations
- PMID: 21957219
- PMCID: PMC3349688
- DOI: 10.1152/jn.00653.2011
Task-level feedback can explain temporal recruitment of spatially fixed muscle synergies throughout postural perturbations
Abstract
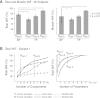
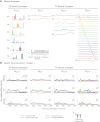
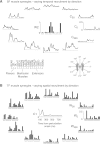
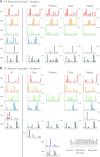
Recent evidence suggests that complex spatiotemporal patterns of muscle activity can be explained with a low-dimensional set of muscle synergies or M-modes. While it is clear that both spatial and temporal aspects of muscle coordination may be low dimensional, constraints on spatial versus temporal features of muscle coordination likely involve different neural control mechanisms. We hypothesized that the low-dimensional spatial and temporal features of muscle coordination are independent of each other. We further hypothesized that in reactive feedback tasks, spatially fixed muscle coordination patterns-or muscle synergies-are hierarchically recruited via time-varying neural commands based on delayed task-level feedback. We explicitly compared the ability of spatially fixed (SF) versus temporally fixed (TF) muscle synergies to reconstruct the entire time course of muscle activity during postural responses to anterior-posterior support-surface translations. While both SF and TF muscle synergies could account for EMG variability in a postural task, SF muscle synergies produced more consistent and physiologically interpretable results than TF muscle synergies during postural responses to perturbations. Moreover, a majority of SF muscle synergies were consistent in structure when extracted from epochs throughout postural responses. Temporal patterns of SF muscle synergy recruitment were well-reconstructed by delayed feedback of center of mass (CoM) kinematics and reproduced EMG activity of multiple muscles. Consistent with the idea that independent and hierarchical low-dimensional neural control structures define spatial and temporal patterns of muscle activity, our results suggest that CoM kinematics are a task variable used to recruit SF muscle synergies for feedback control of balance.
Figures






Similar articles
-
Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies.J Neurophysiol. 2013 Jan;109(1):31-45. doi: 10.1152/jn.00684.2012. Epub 2012 Oct 24. J Neurophysiol. 2013. PMID: 23100133 Free PMC article.
-
A feedback model reproduces muscle activity during human postural responses to support-surface translations.J Neurophysiol. 2008 Feb;99(2):1032-8. doi: 10.1152/jn.01110.2007. Epub 2007 Dec 19. J Neurophysiol. 2008. PMID: 18094102
-
Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors.J Neurophysiol. 2011 Aug;106(2):999-1015. doi: 10.1152/jn.00549.2010. Epub 2011 Jun 8. J Neurophysiol. 2011. PMID: 21653725 Free PMC article.
-
Representation of Muscle Synergies in the Primate Brain.J Neurosci. 2015 Sep 16;35(37):12615-24. doi: 10.1523/JNEUROSCI.4302-14.2015. J Neurosci. 2015. PMID: 26377453 Free PMC article. Review.
-
Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture.Prog Brain Res. 2007;165:299-321. doi: 10.1016/S0079-6123(06)65019-X. Prog Brain Res. 2007. PMID: 17925254 Free PMC article. Review.
Cited by
-
Neuromuscular Control Strategies in Basketball Shooting: Distance-Dependent Analysis of Muscle Synergies.J Sports Sci Med. 2024 Sep 1;23(1):571-580. doi: 10.52082/jssm.2024.571. eCollection 2024 Sep. J Sports Sci Med. 2024. PMID: 39228767 Free PMC article.
-
Increased trial-to-trial similarity and reduced temporal overlap of muscle synergy activation coefficients manifest during learning and with increasing movement proficiency.Sci Rep. 2024 Jul 31;14(1):17638. doi: 10.1038/s41598-024-68515-3. Sci Rep. 2024. PMID: 39085397 Free PMC article.
-
Whole-Body Adaptive Functional Electrical Stimulation Kinesitherapy Can Promote the Restoring of Physiological Muscle Synergies for Neurological Patients.Sensors (Basel). 2022 Feb 13;22(4):1443. doi: 10.3390/s22041443. Sensors (Basel). 2022. PMID: 35214345 Free PMC article.
-
Modeling of muscle forces in humans with and without temporomandibular joint disorders.Orthod Craniofac Res. 2015 Apr;18 Suppl 1(0 1):170-9. doi: 10.1111/ocr.12075. Orthod Craniofac Res. 2015. PMID: 25865546 Free PMC article.
-
Interfacing sensory input with motor output: does the control architecture converge to a serial process along a single channel?Front Comput Neurosci. 2013 May 9;7:55. doi: 10.3389/fncom.2013.00055. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23675342 Free PMC article.
References
-
- Alexandrov A, Frolov A, Massion J. Axial synergies during human upper trunk bending. Exp Brain Res 118: 210–220, 1998 - PubMed
-
- Allum JH, Carpenter MG. A speedy solution for balance and gait analysis: angular velocity measured at the centre of body mass. Curr Opin Neurol 18: 15–21, 2005 - PubMed
-
- Bernstein N. The Coordination and Regulation of Movements. New York: Pergamon, 1967
-
- Bizzi E, Mussa-Ivaldi FA, Giszter SF. Computations underlying the execution of movement: a biological perspective. Science 253: 287–291, 1991 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

