A double-blind randomized phase I clinical trial targeting ALVAC-HIV vaccine to human dendritic cells
- PMID: 21949699
- PMCID: PMC3174939
- DOI: 10.1371/journal.pone.0024254
A double-blind randomized phase I clinical trial targeting ALVAC-HIV vaccine to human dendritic cells
Abstract
Background: We conducted a novel pilot study comparing different delivery routes of ALVAC-HIV (vCP205), a canarypox vaccine containing HIV gene inserts: env, gag and pol. We explored the concept that direct ex vivo targeting of human dendritic cells (DC) would enhance the immune response compared to either conventional intramuscular or intradermal injections of the vaccine alone.
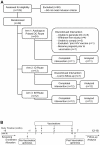
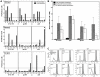
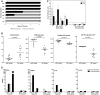
Methodology/principal findings: Healthy HIV-1 uninfected volunteers were administered ALVAC-HIV or placebo by intramuscular injection (i.m.), intradermal injection (i.d.) or subcutaneous injection (s.q.) of autologous ex vivo transfected DC at months 0, 1, 3 and 6. All vaccine delivery routes were well tolerated. Binding antibodies were observed to both the ALVAC vector and HIV-1 gp160 proteins. Modest cellular responses were observed in 2/7 individuals in the DC arm and 1/8 in the i.m. arm as determined by IFN-γ ELISPOT. Proliferative responses were most frequent in the DC arm where 4/7 individuals had measurable responses to multiple HIV-1 antigens. Loading DC after maturation resulted in lower gene expression, but overall better responses to both HIV-1 and control antigens, and were associated with better IL-2, TNF-α and IFN-γ production.
Conclusions/significance: ALVAC-HIV delivered i.m., i.d. or s.q. with autologous ex vivo transfected DC proved to be safe. The DC arm was most immunogenic. Proliferative immune responses were readily detected with only modest cytotoxic CD8 T cell responses. Loading mature DC with the live viral vaccine induced stronger immune responses than loading immature DC, despite increased transgene expression with the latter approach. Volunteers who received the autologous vaccine loaded mature DC developed a broader and durable immune response compared to those vaccinated by conventional routes.
Trial registration: ClinicalTrials.gov NCT00013572.
Conflict of interest statement
Figures
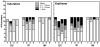

Similar articles
-
Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial.Lancet HIV. 2018 Jul;5(7):e366-e378. doi: 10.1016/S2352-3018(18)30071-7. Epub 2018 Jun 18. Lancet HIV. 2018. PMID: 29898870 Free PMC article. Clinical Trial.
-
A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost.J Acquir Immune Defic Syndr. 2007 Sep 1;46(1):48-55. doi: 10.1097/QAI.0b013e3181354bd7. J Acquir Immune Defic Syndr. 2007. PMID: 17909315 Clinical Trial.
-
Safety and Immunogenicity of a Randomized Phase 1 Prime-Boost Trial With ALVAC-HIV (vCP205) and Oligomeric Glycoprotein 160 From HIV-1 Strains MN and LAI-2 Adjuvanted in Alum or Polyphosphazene.J Infect Dis. 2016 Jun 15;213(12):1946-54. doi: 10.1093/infdis/jiw059. Epub 2016 Feb 11. J Infect Dis. 2016. PMID: 26908741 Free PMC article. Clinical Trial.
-
Dendritic cell based vaccines for HIV infection: the way ahead.Hum Vaccin Immunother. 2013 Nov;9(11):2445-52. doi: 10.4161/hv.25876. Epub 2013 Aug 2. Hum Vaccin Immunother. 2013. PMID: 23912672 Free PMC article. Review.
-
Advances in dendritic cell immunotherapies for HIV-1 infection.Expert Opin Biol Ther. 2014 Nov;14(11):1545-9. doi: 10.1517/14712598.2014.950652. Epub 2014 Aug 21. Expert Opin Biol Ther. 2014. PMID: 25143151 Free PMC article. Review.
Cited by
-
Modulation of Vaccine-Induced CD4 T Cell Functional Profiles by Changes in Components of HIV Vaccine Regimens in Humans.J Virol. 2018 Nov 12;92(23):e01143-18. doi: 10.1128/JVI.01143-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209165 Free PMC article.
-
Poxvirus vectors as HIV/AIDS vaccines in humans.Hum Vaccin Immunother. 2012 Sep;8(9):1192-207. doi: 10.4161/hv.20778. Epub 2012 Aug 21. Hum Vaccin Immunother. 2012. PMID: 22906946 Free PMC article. Review.
-
The evolution of poxvirus vaccines.Viruses. 2015 Apr 7;7(4):1726-803. doi: 10.3390/v7041726. Viruses. 2015. PMID: 25853483 Free PMC article. Review.
-
The Immunology of a Healing Response in Cutaneous Leishmaniasis Treated with Localized Heat or Systemic Antimonial Therapy.PLoS Negl Trop Dis. 2015 Oct 20;9(10):e0004178. doi: 10.1371/journal.pntd.0004178. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26485398 Free PMC article.
-
Harnessing T-Cells for Enhanced Vaccine Development against Viral Infections.Vaccines (Basel). 2024 Apr 29;12(5):478. doi: 10.3390/vaccines12050478. Vaccines (Basel). 2024. PMID: 38793729 Free PMC article. Review.
References
-
- UNAIDS. 2009. 2009 AIDS Epidemic Update.
-
- Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464:224–231. - PubMed
-
- Database of AIDS Vaccine Candidates in Clinical Trials. IAVI.
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med 2009 - PubMed
-
- Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

