The TRAF-associated protein TANK facilitates cross-talk within the IkappaB kinase family during Toll-like receptor signaling
- PMID: 21949249
- PMCID: PMC3193242
- DOI: 10.1073/pnas.1114194108
The TRAF-associated protein TANK facilitates cross-talk within the IkappaB kinase family during Toll-like receptor signaling
Abstract
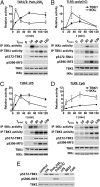
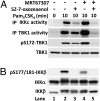
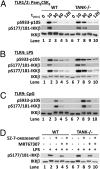
Toll-like receptor (TLR) ligands that signal via TIR-domain-containing adapter-inducing IFNβ (TRIF) activate the IκB kinase (IKK)-related kinases, TRAF associated NFκB activator (TANK)-binding kinase-1 (TBK1) and IKKε, which then phosphorylate IRF3 and induce the production of IFNβ. Here we show that TBK1 and IKKε are also activated by TLR ligands that signal via MyD88. Notably, the activation of IKKε is rapid, transient, and it precedes a more prolonged activation of TBK1. The MyD88- and TRIF-dependent signaling pathways activate the IKK-related kinases by two signaling pathways. One is mediated by the canonical IKKs, whereas the other culminates in the autoactivation of the IKK-related kinases. Once activated, TBK1/IKKε then phosphorylate and inhibit the canonical IKKs. The negative regulation of the canonical IKKs by the IKK-related kinases occurs in both the TRIF- and MyD88-dependent TLR pathways, whereas IRF3 phosphorylation is restricted to the TRIF-dependent signaling pathway. We have discovered that the activation of IKKε is abolished, the activation of TBK1 is reduced, and the interaction between the IKK-related kinases and the canonical IKKs is suppressed in TANK(-/-) macrophages, preventing the IKK-related kinases from negatively regulating the canonical IKKs. In contrast, IRF3 phosphorylation and IFNβ production was normal in TANK(-/-) macrophages. Our results demonstrate a key role for TANK in enabling the canonical IKKs and the IKK-related kinases to regulate each other, which is required to limit the strength of TLR signaling and ultimately, prevent autoimmunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



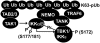
Similar articles
-
The IkappaB kinase family phosphorylates the Parkinson's disease kinase LRRK2 at Ser935 and Ser910 during Toll-like receptor signaling.PLoS One. 2012;7(6):e39132. doi: 10.1371/journal.pone.0039132. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723946 Free PMC article.
-
Identification of TBK1 complexes required for the phosphorylation of IRF3 and the production of interferon β.Biochem J. 2017 Mar 15;474(7):1163-1174. doi: 10.1042/BCJ20160992. Biochem J. 2017. PMID: 28159912 Free PMC article.
-
The role of TBK1 and IKKε in the expression and activation of Pellino 1.Biochem J. 2011 Mar 15;434(3):537-48. doi: 10.1042/BJ20101421. Biochem J. 2011. PMID: 21204785 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated?Trends Biochem Sci. 2008 Apr;33(4):171-80. doi: 10.1016/j.tibs.2008.01.002. Epub 2008 Mar 18. Trends Biochem Sci. 2008. PMID: 18353649 Review.
Cited by
-
IκB kinase ε (IKKε): a therapeutic target in inflammation and cancer.Biochem Pharmacol. 2013 Apr 1;85(7):873-80. doi: 10.1016/j.bcp.2013.01.007. Epub 2013 Jan 17. Biochem Pharmacol. 2013. PMID: 23333767 Free PMC article. Review.
-
Essential role for IKKβ in production of type 1 interferons by plasmacytoid dendritic cells.J Biol Chem. 2012 Jun 1;287(23):19216-28. doi: 10.1074/jbc.M112.345405. Epub 2012 Apr 16. J Biol Chem. 2012. PMID: 22511786 Free PMC article.
-
Transcriptional and functional characterization of neonatal circulating Innate Lymphoid Cells.Stem Cells Transl Med. 2021 Jun;10(6):867-882. doi: 10.1002/sctm.20-0300. Epub 2021 Jan 21. Stem Cells Transl Med. 2021. PMID: 33475258 Free PMC article.
-
An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice.Nat Med. 2013 Mar;19(3):313-21. doi: 10.1038/nm.3082. Epub 2013 Feb 10. Nat Med. 2013. PMID: 23396211 Free PMC article.
-
TBK1 Is a Synthetic Lethal Target in Cancer with VHL Loss.Cancer Discov. 2020 Mar;10(3):460-475. doi: 10.1158/2159-8290.CD-19-0837. Epub 2019 Dec 6. Cancer Discov. 2020. PMID: 31810986 Free PMC article.
References
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. - PubMed
-
- Kanayama A, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. - PubMed
-
- Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

