Cdc15 integrates Tem1 GTPase-mediated spatial signals with Polo kinase-mediated temporal cues to activate mitotic exit
- PMID: 21937712
- PMCID: PMC3185966
- DOI: 10.1101/gad.17257711
Cdc15 integrates Tem1 GTPase-mediated spatial signals with Polo kinase-mediated temporal cues to activate mitotic exit
Abstract
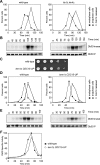
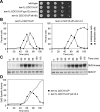
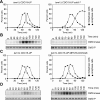
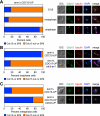
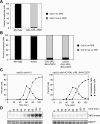
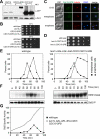
In budding yeast, a Ras-like GTPase signaling cascade known as the mitotic exit network (MEN) promotes exit from mitosis. To ensure the accurate execution of mitosis, MEN activity is coordinated with other cellular events and restricted to anaphase. The MEN GTPase Tem1 has been assumed to be the central switch in MEN regulation. We show here that during an unperturbed cell cycle, restricting MEN activity to anaphase can occur in a Tem1 GTPase-independent manner. We found that the anaphase-specific activation of the MEN in the absence of Tem1 is controlled by the Polo kinase Cdc5. We further show that both Tem1 and Cdc5 are required to recruit the MEN kinase Cdc15 to spindle pole bodies, which is both necessary and sufficient to induce MEN signaling. Thus, Cdc15 functions as a coincidence detector of two essential cell cycle oscillators: the Polo kinase Cdc5 synthesis/degradation cycle and the Tem1 G-protein cycle. The Cdc15-dependent integration of these temporal (Cdc5 and Tem1 activity) and spatial (Tem1 activity) signals ensures that exit from mitosis occurs only after proper genome partitioning.
Figures
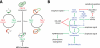
Similar articles
-
A noncanonical GTPase signaling mechanism controls exit from mitosis in budding yeast.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2413873121. doi: 10.1073/pnas.2413873121. Epub 2024 Oct 30. Proc Natl Acad Sci U S A. 2024. PMID: 39475649 Free PMC article.
-
Regulation of the mitotic exit protein kinases Cdc15 and Dbf2.Mol Biol Cell. 2001 Oct;12(10):2961-74. doi: 10.1091/mbc.12.10.2961. Mol Biol Cell. 2001. PMID: 11598184 Free PMC article.
-
Mitotic exit control: a space and time odyssey.Curr Biol. 2011 Oct 25;21(20):R857-9. doi: 10.1016/j.cub.2011.09.023. Curr Biol. 2011. PMID: 22032192 Review.
-
A novel functional domain of Cdc15 kinase is required for its interaction with Tem1 GTPase in Saccharomyces cerevisiae.Genetics. 2001 Apr;157(4):1437-50. doi: 10.1093/genetics/157.4.1437. Genetics. 2001. PMID: 11290702 Free PMC article.
-
Coupling spindle position with mitotic exit in budding yeast: The multifaceted role of the small GTPase Tem1.Small GTPases. 2015 Oct 2;6(4):196-201. doi: 10.1080/21541248.2015.1109023. Small GTPases. 2015. PMID: 26507466 Free PMC article. Review.
Cited by
-
Meiotic Cytokinesis in Saccharomyces cerevisiae: Spores That Just Need Closure.J Fungi (Basel). 2024 Feb 6;10(2):132. doi: 10.3390/jof10020132. J Fungi (Basel). 2024. PMID: 38392804 Free PMC article. Review.
-
Functions and regulation of the Polo-like kinase Cdc5 in the absence and presence of DNA damage.Curr Genet. 2018 Feb;64(1):87-96. doi: 10.1007/s00294-017-0727-2. Epub 2017 Aug 2. Curr Genet. 2018. PMID: 28770345 Free PMC article. Review.
-
Unifying the mechanism of mitotic exit control in a spatiotemporal logical model.PLoS Biol. 2020 Nov 12;18(11):e3000917. doi: 10.1371/journal.pbio.3000917. eCollection 2020 Nov. PLoS Biol. 2020. PMID: 33180788 Free PMC article.
-
The regulation of Net1/Cdc14 by the Hog1 MAPK upon osmostress unravels a new mechanism regulating mitosis.Cell Cycle. 2020 Sep;19(17):2105-2118. doi: 10.1080/15384101.2020.1804222. Epub 2020 Aug 14. Cell Cycle. 2020. PMID: 32794416 Free PMC article. Review.
-
A common molecular mechanism underlies the role of Mps1 in chromosome biorientation and the spindle assembly checkpoint.EMBO Rep. 2020 Jun 4;21(6):e50257. doi: 10.15252/embr.202050257. Epub 2020 Apr 19. EMBO Rep. 2020. PMID: 32307893 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
