Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism
- PMID: 21932346
- PMCID: PMC4118835
- DOI: 10.1002/jbmr.508
Proteoglycan 4: a dynamic regulator of skeletogenesis and parathyroid hormone skeletal anabolism
Abstract
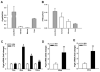
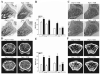
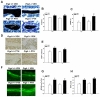
Proteoglycan 4 (Prg4), known for its lubricating and protective actions in joints, is a strong candidate regulator of skeletal homeostasis and parathyroid hormone (PTH) anabolism. Prg4 is a PTH-responsive gene in bone and liver. Prg4 null mutant mice were used to investigate the impact of proteoglycan 4 on skeletal development, remodeling, and PTH anabolic actions. Young Prg4 mutant and wild-type mice were administered intermittent PTH(1-34) or vehicle daily from 4 to 21 days. Young Prg4 mutant mice had decreased growth plate hypertrophic zones, trabecular bone, and serum bone formation markers versus wild-type mice, but responded with a similar anabolic response to PTH. Adult Prg4 mutant and wild-type mice were administered intermittent PTH(1-34) or vehicle daily from 16 to 22 weeks. Adult Prg4 mutant mice had decreased trabecular and cortical bone, and blunted PTH-mediated increases in bone mass. Joint range of motion and animal mobility were lower in adult Prg4 mutant versus wild-type mice. Adult Prg4 mutant mice had decreased marrow and liver fibroblast growth factor 2 (FGF-2) mRNA and reduced serum FGF-2, which were normalized by PTH. A single dose of PTH decreased the PTH/PTHrP receptor (PPR), and increased Prg4 and FGF-2 to a similar extent in liver and bone. Proteoglycan 4 supports endochondral bone formation and the attainment of peak trabecular bone mass, and appears to support skeletal homeostasis indirectly by protecting joint function. Bone- and liver-derived FGF-2 likely regulate proteoglycan 4 actions supporting trabeculae formation. Blunted PTH anabolic responses in adult Prg4 mutant mice are associated with altered biomechanical impact secondary to joint failure.
Copyright © 2012 American Society for Bone and Mineral Research.
Figures

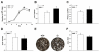

Similar articles
-
Proteoglycan 4, a novel immunomodulatory factor, regulates parathyroid hormone actions on hematopoietic cells.Am J Pathol. 2011 Nov;179(5):2431-42. doi: 10.1016/j.ajpath.2011.07.032. Epub 2011 Sep 21. Am J Pathol. 2011. PMID: 21939632 Free PMC article.
-
Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids.J Bone Miner Res. 2011 Jun;26(6):1197-206. doi: 10.1002/jbmr.312. J Bone Miner Res. 2011. PMID: 21611962 Free PMC article.
-
Impact of proteoglycan-4 and parathyroid hormone on articular cartilage.J Orthop Res. 2013 Feb;31(2):183-90. doi: 10.1002/jor.22207. Epub 2012 Aug 15. J Orthop Res. 2013. PMID: 22898906 Free PMC article.
-
[Morphological analysis of bone dynamics and metabolic bone disease. Effects of parathyroid hormone on bone tissue].Clin Calcium. 2011 Apr;21(4):575-81. Clin Calcium. 2011. PMID: 21447925 Review. Japanese.
-
[Parathyroid and bone. Effect of parathyroid hormone on bone quality].Clin Calcium. 2007 Dec;17(12):1858-64. Clin Calcium. 2007. PMID: 18057661 Review. Japanese.
Cited by
-
Inflammatory bone loss associated with MFG-E8 deficiency is rescued by teriparatide.FASEB J. 2018 Jul;32(7):3730-3741. doi: 10.1096/fj.201701238R. Epub 2018 Feb 22. FASEB J. 2018. PMID: 29475373 Free PMC article.
-
Tristetraprolin Is Required for Alveolar Bone Homeostasis.J Dent Res. 2018 Jul;97(8):946-953. doi: 10.1177/0022034518756889. Epub 2018 Mar 7. J Dent Res. 2018. PMID: 29514008 Free PMC article.
-
Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis.J Biol Chem. 2014 Aug 29;289(35):24560-72. doi: 10.1074/jbc.M114.571620. Epub 2014 Jul 8. J Biol Chem. 2014. PMID: 25006249 Free PMC article.
-
Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health.Sci Rep. 2017 Jul 18;7(1):5747. doi: 10.1038/s41598-017-06126-x. Sci Rep. 2017. PMID: 28720797 Free PMC article.
-
TGF-β Activity of a Demineralized Bone Matrix.Int J Mol Sci. 2021 Jan 11;22(2):664. doi: 10.3390/ijms22020664. Int J Mol Sci. 2021. PMID: 33440877 Free PMC article.
References
-
- Kousteni S, Bilezikian JP. Cellular actions of parathyroid hormone. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of bone biology. 3rd Elsevier Press; Burlington, MA: 2008. pp. 639–56.
-
- Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, Garcia-Hernandez PA, Recknor CP, Einhorn TA, Dalsky GP, Mitlak BH, Fierlinger A, Lakshmanan MC. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–14. - PubMed
-
- Hurley MM, Tetradis S, Huang YF, Hock J, Kream BE, Raisz LG. Parathyroid hormone regulates the expression of fibroblast growth factor-2 mRNA and fibroblast growth factor receptor mRNA in osteoblastic cells. J Bone Miner Res. 1999;14:776–83. - PubMed
-
- Watson P, Lazowski D, Han V, Fraher L, Steer B, Hodsman A. Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor 1 gene expression in ovariectomized rats. Bone. 1995;16:357–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

