Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas
- PMID: 21927024
- PMCID: PMC3243769
- DOI: 10.1038/onc.2011.405
Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas
Abstract
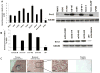
Tumors are thought to be sustained by a reservoir of self-renewing cells, termed tumor-initiating cells or cancer stem cells. Osteosarcomas are high-grade sarcomas derived from osteoblast progenitor cells and are the most common pediatric bone malignancy. In this report we show that the stem cell transcription factor Sox2 is highly expressed in human and murine osteosarcoma (mOS) cell lines as well as in the tumor samples. Osteosarcoma cells have increased ability to grow in suspension as osteospheres, that are greatly enriched in expression of Sox2 and the stem cell marker, Sca-1. Depletion of Sox2 by short-hairpin RNAs in independent mOS-derived cells drastically reduces their transformed properties in vitro and their ability to form tumors. Sox2-depleted osteosarcoma cells can no longer form osteospheres and differentiate into mature osteoblasts. Concomitantly, they exhibit decreased Sca-1 expression and upregulation of the Wnt signaling pathway. Thus, despite other mutations, these cells maintain a requirement for Sox2 for tumorigenicity. Our data indicate that Sox2 is required for osteosarcoma cell self renewal, and that Sox2 antagonizes the pro-differentiation Wnt pathway that can in turn reduce Sox2 expression. These studies define Sox2 as a survival factor and a novel biomarker of self renewal in osteosarcomas, and support a tumor suppressive role for the Wnt pathway in tumors of mesenchymal origin. Our findings could provide the basis for novel therapeutic strategies based on inhibiting Sox2 or enhancing Wnt signaling for the treatment of osteosarcomas.
Conflict of interest statement
Figures




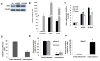

Similar articles
-
Sox2 is required for tumor development and cancer cell proliferation in osteosarcoma.Oncogene. 2018 Aug;37(33):4626-4632. doi: 10.1038/s41388-018-0292-2. Epub 2018 May 10. Oncogene. 2018. PMID: 29743593 Free PMC article.
-
Aberrant Wnt/β-catenin signaling and elevated expression of stem cell proteins are associated with osteosarcoma side population cells of high tumorigenicity.Mol Med Rep. 2015 Oct;12(4):5042-8. doi: 10.3892/mmr.2015.4025. Epub 2015 Jul 2. Mol Med Rep. 2015. PMID: 26134785 Free PMC article.
-
Perspectives on cancer stem cells in osteosarcoma.Cancer Lett. 2013 Sep 10;338(1):158-67. doi: 10.1016/j.canlet.2012.05.028. Epub 2012 May 29. Cancer Lett. 2013. PMID: 22659734 Free PMC article. Review.
-
miR-34a is downregulated in human osteosarcoma stem-like cells and promotes invasion, tumorigenic ability and self-renewal capacity.Mol Med Rep. 2017 Apr;15(4):1631-1637. doi: 10.3892/mmr.2017.6187. Epub 2017 Feb 9. Mol Med Rep. 2017. Retraction in: Mol Med Rep. 2024 May;29(5):72. doi: 10.3892/mmr.2024.13196 PMID: 28260055 Free PMC article. Retracted.
-
SOX2 and p53 Expression Control Converges in PI3K/AKT Signaling with Versatile Implications for Stemness and Cancer.Int J Mol Sci. 2020 Jul 11;21(14):4902. doi: 10.3390/ijms21144902. Int J Mol Sci. 2020. PMID: 32664542 Free PMC article. Review.
Cited by
-
Prognostic implication of SOX2 expression in small intestinal adenocarcinoma.Virchows Arch. 2021 Jun;478(6):1049-1060. doi: 10.1007/s00428-020-02946-x. Epub 2020 Oct 25. Virchows Arch. 2021. PMID: 33103210 Free PMC article.
-
RIF1 promotes human epithelial ovarian cancer growth and progression via activating human telomerase reverse transcriptase expression.J Exp Clin Cancer Res. 2018 Aug 3;37(1):182. doi: 10.1186/s13046-018-0854-8. J Exp Clin Cancer Res. 2018. PMID: 30075819 Free PMC article.
-
Targeting the SOX2/CDP protein complex with a peptide suppresses the malignant progression of esophageal squamous cell carcinoma.Cell Death Discov. 2023 Oct 27;9(1):399. doi: 10.1038/s41420-023-01693-7. Cell Death Discov. 2023. PMID: 37891174 Free PMC article.
-
Characterization of cancer stem-like cells derived from mouse induced pluripotent stem cells transformed by tumor-derived extracellular vesicles.J Cancer. 2014 Jul 5;5(7):572-84. doi: 10.7150/jca.8865. eCollection 2014. J Cancer. 2014. PMID: 25057308 Free PMC article.
-
Sox2-dependent inhibition of p21 is associated with poor prognosis of endometrial cancer.Cancer Sci. 2017 Apr;108(4):632-640. doi: 10.1111/cas.13196. Epub 2017 Apr 19. Cancer Sci. 2017. PMID: 28188685 Free PMC article.
References
-
- Adams JM, Kelly PN, Dakic A, Carotta S, Nutt SL, Strasser A. Role of “cancer stem cells” and cell survival in tumor development and maintenance. Cold Spring Harb Symp Quant Biol. 2008;73:451–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

