Pleiotropic functions of EAPII/TTRAP/TDP2: cancer development, chemoresistance and beyond
- PMID: 21926483
- PMCID: PMC3233625
- DOI: 10.4161/cc.10.19.17763
Pleiotropic functions of EAPII/TTRAP/TDP2: cancer development, chemoresistance and beyond
Abstract
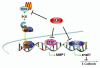
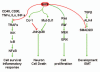
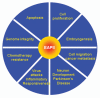
EAPII (also called TTRAP, TDP2), a protein identified a decade ago, has recently been shown to function as an oncogenic factor. This protein was also proven to be the first 5'- tyrosyl-DNA phosphodiesterase. EAPII has been demonstrated to have promiscuous protein associations, broad responsiveness to various extracellular signals, and pleiotropic functions in the development of human diseases including cancer and neurodegenerative disease. Emerging data suggest that EAPII is a multi-functional protein: EAPII repairs enzyme (topoisomerase)-mediated DNA damage by removing phosphotyrosine from DNA adducts; EAPII is involved in multiple signal transduction pathways such as TNF-TNFR, TGFβ and MAPK, and EAPII is responsive to immune defense, inflammatory response, virus infection and DNA toxins (chemo or radiation therapy). This review focuses on the current understanding of EAPII biology and its potential relations to many aspects of cancer development, including chromosome instability, tumorigenesis, tumor metastasis and chemoresistance, suggesting it as a potential target for intervention in cancer and other human diseases.
© 2011 Landes Bioscience
Figures

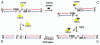
Similar articles
-
Biochemical characterization of human tyrosyl-DNA phosphodiesterase 2 (TDP2/TTRAP): a Mg(2+)/Mn(2+)-dependent phosphodiesterase specific for the repair of topoisomerase cleavage complexes.J Biol Chem. 2012 Aug 31;287(36):30842-52. doi: 10.1074/jbc.M112.393983. Epub 2012 Jul 20. J Biol Chem. 2012. PMID: 22822062 Free PMC article.
-
TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1.Nucleic Acids Res. 2012 Sep 1;40(17):8371-80. doi: 10.1093/nar/gks622. Epub 2012 Jun 26. Nucleic Acids Res. 2012. PMID: 22740648 Free PMC article.
-
Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2).DNA Repair (Amst). 2014 Jul;19:114-29. doi: 10.1016/j.dnarep.2014.03.020. Epub 2014 May 22. DNA Repair (Amst). 2014. PMID: 24856239 Free PMC article. Review.
-
A human 5'-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage.Nature. 2009 Oct 1;461(7264):674-8. doi: 10.1038/nature08444. Nature. 2009. PMID: 19794497
-
Tyrosyl-DNA phosphodiesterases: rescuing the genome from the risks of relaxation.Nucleic Acids Res. 2018 Jan 25;46(2):520-537. doi: 10.1093/nar/gkx1219. Nucleic Acids Res. 2018. PMID: 29216365 Free PMC article. Review.
Cited by
-
The repair of topoisomerase 2 cleavage complexes in Arabidopsis.Plant Cell. 2022 Jan 20;34(1):287-301. doi: 10.1093/plcell/koab228. Plant Cell. 2022. PMID: 34524446 Free PMC article.
-
Divergent Requirement for a DNA Repair Enzyme during Enterovirus Infections.mBio. 2015 Dec 29;7(1):e01931-15. doi: 10.1128/mBio.01931-15. mBio. 2015. PMID: 26715620 Free PMC article.
-
An RNA virus hijacks an incognito function of a DNA repair enzyme.Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14634-9. doi: 10.1073/pnas.1208096109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908287 Free PMC article.
-
Novel TDP2-ubiquitin interactions and their importance for the repair of topoisomerase II-mediated DNA damage.Nucleic Acids Res. 2016 Dec 1;44(21):10201-10215. doi: 10.1093/nar/gkw719. Epub 2016 Aug 19. Nucleic Acids Res. 2016. PMID: 27543075 Free PMC article.
-
Topoisomerase-Mediated DNA Damage in Neurological Disorders.Front Aging Neurosci. 2021 Nov 25;13:751742. doi: 10.3389/fnagi.2021.751742. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34899270 Free PMC article. Review.
References
-
- Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. - PubMed
-
- Pype S, Declercq W, Ibrahimi A, Michiels C, Van Rietschoten JG, Dewulf N, et al. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappaB activation. J Biol Chem. 2000;275:18586–18593. doi: 10.1074/jbc.M000531200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
