Single cell wound repair: Dealing with life's little traumas
- PMID: 21922041
- PMCID: PMC3173964
- DOI: 10.4161/bioa.1.3.17091
Single cell wound repair: Dealing with life's little traumas
Abstract
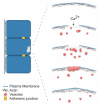
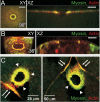
Cell wounding is a common event in the life of many cell types, and the capacity of the cell to repair day-to-day wear-and-tear injuries, as well as traumatic ones, is fundamental for maintaining tissue integrity. Cell wounding is most frequent in tissues exposed to high levels of stress. Survival of such plasma membrane disruptions requires rapid resealing to prevent the loss of cytosolic components, to block Ca(2+) influx and to avoid cell death. In addition to patching the torn membrane, plasma membrane and cortical cytoskeleton remodeling are required to restore cell function. Although a general understanding of the cell wound repair process is in place, the underlying mechanisms of each step of this response are not yet known. We have developed a model to study single cell wound repair using the early Drosophila embryo. Our system combines genetics and live imaging tools, allowing us to dissect in vivo the dynamics of the single cell wound response. We have shown that cell wound repair in Drosophila requires the coordinated activities of plasma membrane and cytoskeleton components. Furthermore, we identified an unexpected role for E-cadherin as a link between the contractile actomyosin ring and the newly formed plasma membrane plug.
Figures

Similar articles
-
Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling.J Cell Biol. 2011 May 2;193(3):455-64. doi: 10.1083/jcb.201011018. Epub 2011 Apr 25. J Cell Biol. 2011. PMID: 21518790 Free PMC article.
-
Actin Cytoskeletal Dynamics in Single-Cell Wound Repair.Int J Mol Sci. 2021 Oct 8;22(19):10886. doi: 10.3390/ijms221910886. Int J Mol Sci. 2021. PMID: 34639226 Free PMC article. Review.
-
Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string.J Cell Sci. 2012 Dec 15;125(Pt 24):5984-97. doi: 10.1242/jcs.109066. Epub 2012 Oct 4. J Cell Sci. 2012. PMID: 23038780 Free PMC article.
-
Into the breach: how cells cope with wounds.Open Biol. 2018 Oct 3;8(10):180135. doi: 10.1098/rsob.180135. Open Biol. 2018. PMID: 30282661 Free PMC article. Review.
-
Cytoskeleton responses in wound repair.Cell Mol Life Sci. 2012 Aug;69(15):2469-83. doi: 10.1007/s00018-012-0928-2. Epub 2012 Feb 15. Cell Mol Life Sci. 2012. PMID: 22349211 Free PMC article. Review.
Cited by
-
Wrangling Actin Assemblies: Actin Ring Dynamics during Cell Wound Repair.Cells. 2022 Sep 6;11(18):2777. doi: 10.3390/cells11182777. Cells. 2022. PMID: 36139352 Free PMC article. Review.
-
Microphysiological systems for the modeling of wound healing and evaluation of pro-healing therapies.J Mater Chem B. 2020 Aug 19;8(32):7062-7075. doi: 10.1039/d0tb00544d. J Mater Chem B. 2020. PMID: 32756718 Free PMC article. Review.
-
Fluxes of Ca2+ and K+ are required for the listeriolysin O-dependent internalization pathway of Listeria monocytogenes.Infect Immun. 2014 Mar;82(3):1084-91. doi: 10.1128/IAI.01067-13. Epub 2013 Dec 23. Infect Immun. 2014. PMID: 24366251 Free PMC article.
-
From damage response to action potentials: early evolution of neural and contractile modules in stem eukaryotes.Philos Trans R Soc Lond B Biol Sci. 2016 Jan 5;371(1685):20150043. doi: 10.1098/rstb.2015.0043. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 26598726 Free PMC article. Review.
-
Cell-type-specific roles of Na+/K+ ATPase subunits in Drosophila auditory mechanosensation.Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):181-6. doi: 10.1073/pnas.1208866110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2013. PMID: 23248276 Free PMC article.
References
-
- Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995;76:927–934. - PubMed
-
- McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96:1238–1248. - PubMed
-
- McNeil PL, Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci. 1990;96:549–556. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
